
Photochemistry : Dienes and Polyenes
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 2-9-2018
2-9-2018
 3082
3082
Photochemistry : Dienes and Polyenes
Acyclic conjugated dienes, trienes and polyenes absorb strongly in the 220 to 300 nm region of the near ultraviolet. The resultant singlet excited states undergo a variety of reactions, as shown in the following diagram for 1,3,5-hexatriene and two 2,5-dialkyl derivatives. Two configurational isomers (E & Z) are possible, together with three coplanar s-cis and s-trans conformations for each (the cEt structure is not drawn). The E-configuration is normally more stable than its Z-isomer, and for R=H or CH3 the elongated tEt conformation is preferred. The various conformers of both the E & Z-isomers are rapidly interconverted at room temperature, the equilibrium composition depending on the size of substituents such as R. The light induced isomerization about the central double bond of the trienes (e.g. E→Z) is an example of OBF.
Now, electronic π → π* excitation of any triene increases the π-bonding between carbons 2 & 3 as well as 4 & 5, as may be seen in the HOMO (π3) and LUMO (π4*) molecular orbitals of the parent triene. Taken together with the very short lifetimes of these excited states (≤10 nsec), this suggests that photochemical products should reflect the rotamer composition of the ground state. Put another way, excited state rotamers are not expected to equilibrate prior to reaction, the NEER principle (Non-Equilibration of Excited Rotamers). An example of NEER is found in the absence of 1,3-cyclohexadiene among the photoproducts from the parent triene (R=H), compared with its increased quantum yield as R is changed to methyl and then tert-butyl (increased cZc concentration).
For E as well as Z-trienes, reactivity parallels an increase in the population of non-planar s-cis rotamers. Furthermore, 1,2-divinylcyclopentene (drawn in the gray-shaded box) is photochemically unreactive and exhibits fluorescence in solution, demonstrating that twisting about the central (3,4-) double bond of the triene is an essential factor in these reactions.
Irradiation of trans,cis,cis-1,3,5-cyclononatriene, shown at 254nm light establishes a 60:40 photoequilibrium with the cis-fused bicyclononadiene on its right. This equilibrium is shifted to the right by longer wavelength 300nm light, which also induces further electrocyclization to tricyclo[4.3.0.0]non-3-ene. As a rule, cyclohexadienes absorb light at longer wavelength than similar non-cyclic hexatrienes. Higher temperatures permit the thermal electrocyclization of trans,cis,cis-1,3,5-cyclononatriene to the trans-fused bicyclononadiene on its left.

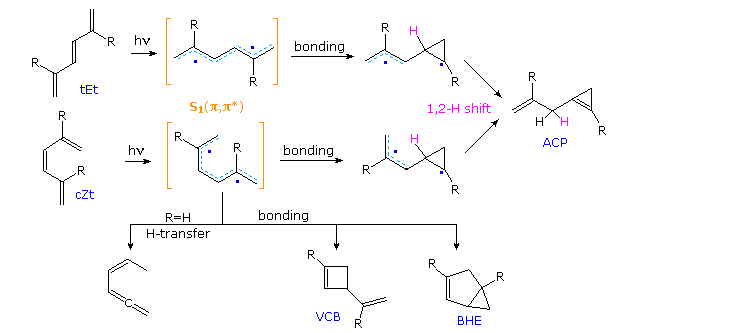
Finally, diradical mechanisms for the formation of allylcyclopropene (ACP), vinylcyclobutene (VCB) and bicyclohexene (BHE) products will be shown above by clicking on the diagram. ACP may be formed from either the tEt or cZt excited states, but VCB and BHE require the latter rotamer. The MVCP product on the left is unique to R = CH3.
As noted above, the reduced bond order of a formal double bond in its first excited state leads to the OBF mechanism for isomerization in fluid solution. The torsional movement in this change is sensitive to the viscosity of the solution and to other structural constraints that may exist. Thus, the facile isomerization and weak fluorescence, typical for trans-stilbene in fluid solutions, change to low isomerization and strong fluorescence in rigid media at low temperatures. Here, the rapid radiationless deactivation of the excited state by OBF is impeded and a normally non-fluorescent compound becomes fluorescent.
A more complex, higher energy motion that achieves double bond isomerization in restrictive environments has been described and named the Hula-Twist. As shown for a cis-stilbene derivative in the following diagram, the OBF process involves a 180º flip of the aryl group on the right side of the double bond. By contrast, the HT process proceeds by simultaneous rotation of a small C–H moiety (light blue ellipse) about the double bond and an adjacent single bond (both colored brown). This not only converts the cis-isomer to its trans-configuration, but also effects a conformational change (note the orientation of the red hydrogen relative to the aryl substituent on its left).
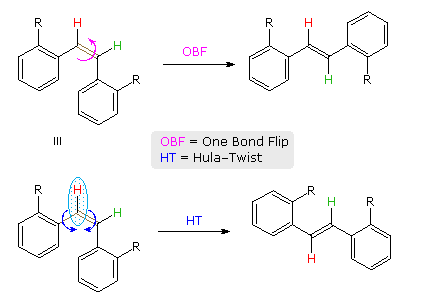
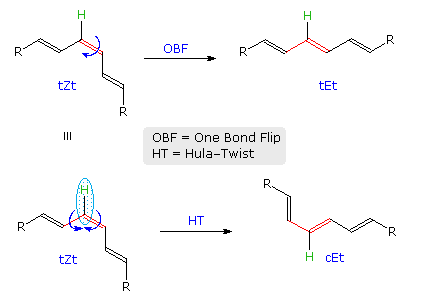
The difference between OBF and HT isomerization of a Z to E-triene will be shown above by clicking on the diagram. It has been proposed that photoisomerization of biologically important polyenes, such as retinal and calciferol may occur by the HT mechanism because translocation of a single H-atom is less volume demanding than the turning over of one-half the molecule, as required by the OBF process. This would be especially important when the chromophore is encapsulated in a protein or bilayer membrane.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


