
Photoenolization
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 2-9-2018
2-9-2018
 1824
1824
Photoenolization
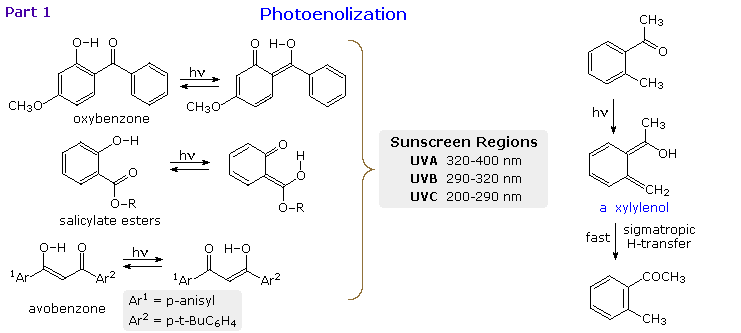
When the reactive carbonyl function and a γ-hydrogen are conjugated via an aromatic ring or double bond, the 1,4-diradical created by hydrogen abstraction quickly relaxes to a conjugated enol tautomer. This is illustrated in the following diagram for a β-hydroxyl substituent, examples on the left, and an ortho-tolyl ketone on the right. If an aromatic ring has been disrupted by the photoenolization, as in all the cases but avobenzone (lower left), the enoltautomer is unstable and rapidly reverts to the initial aromatic carbonyl compound. This might appear to be a useless transformation, but it finds practical application as a sunscreen ingredient. The three compounds on the left are examples of current sunscreen components. Oxybenzone screens UVA, salicylate esters (R=C8 to C10) screen UVB and avobenzone screens both.
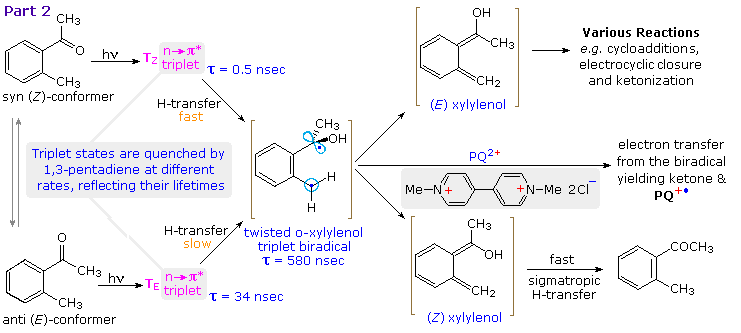
Careful studies of the photoenolization of ortho-alkyl benzophenones and acetophenones have enhanced our understanding of this deceptively simple transformation. By clicking on the diagram the behavior of ortho-methylacetophenone will be elaborated. Excitation of the anti and syn mixture of this ketone leads to two stereoisomeric n→ π* triplet states, TE and TZ respectively. The former has a much longer lifetime (τ) than the latter since it must undergo a conformational change before γ-H abstraction from the ortho-methyl substituent can take place. In both instances a twisted triplet biradical is formed. This biradical may be intercepted by electron transfer to PQ2+ followed by rapid proton loss. This event is easily monitored by the intense blue color of the PQ+• radical cation, and serves to set the lifetime of the biradical at 580 nsec. Relaxation of the biradical leads to a mixture of (E) and (Z)-xylylenols, the latter undergoing rapid H-transfer back to the starting ketone. (Z)-Xylylenol formation directly from a syn n→ π* singlet state is possible and would not be detected.
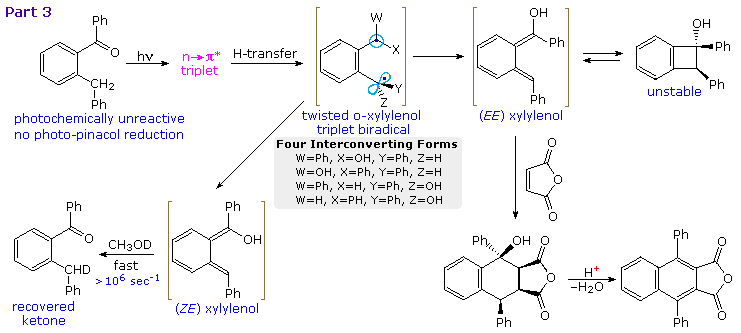
Depending on the reaction conditions, (E)-xylylenols may undergo a variety of reactions or slowly relax back to the initial ketone. Above diagram will show examples of other reactions (Part 3). Unlike benzophenone itself, the ortho substituted compound in the upper left corner does not undergo any pinacol reduction. Instead, γ-H abstraction produces a mixture of interconverting twisted biradicals which decay predominantly to (E,E) and (Z,E)-xylylenols. Only the former is sufficiently long lived to yield distinctive products. At temperatures below 0 ºC conrotatory electrocyclization forms cis-1,2-diphenylbenzocyclobutenol in high yield. At room temperature or above, conrotatory ring opening regenerates the (E,E)-xylylenol which may be trapped by cycloaddition to maleic anhydride.
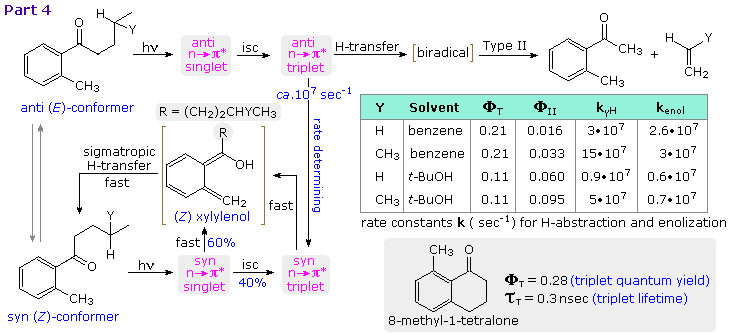
Competition between photoenolization and type II cleavage has been explored in the manner shown above, by clicking on the diagram a third time (Part 4). The syn conformer of butyl (and isoamyl) o-tolyl ketone gives exclusive photoenolization, 60% from the n → π* singlet state, the corresponding triplet state undergoing xylylenol formation faster than type II cleavage. The anti conformer reacts exclusively from the triplet state, which either isomerizes to the syn triplet or generates the biradical precursor to type II products. As noted in the previous section, t-butanol favors type II cleavage. The 8-methyl-1-tetralone served as a reference molecule, constrained with the carbonyl oxygen syn to the methyl substituent.
 الاكثر قراءة في كيمياء عامة
الاكثر قراءة في كيمياء عامة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


