
Imine and Enamine Intermediates
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 5-8-2018
5-8-2018
 1996
1996
Imine and Enamine Intermediates
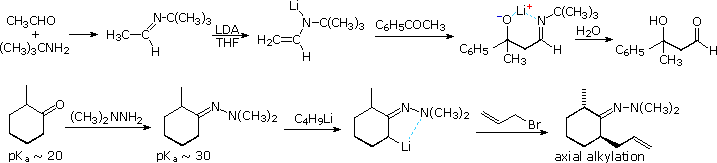
Examples of imine and enamine analogs of enolate species are well known. The following diagram gives two examples of metalated imine and hydrazone intermediates in carbon bond forming reactions at an α-carbon. The first is an aldol reaction which would be difficult to accomplish directly. Although the α-hydrogen acidity of aldehydes and ketones do not differ appreciably, aldehydes are much better enolate acceptors than are ketones. By using a preformed tert-butylimine of acetaldehyde as the enolate donor source, the ketone is forced to react as a nucleophile acceptor. Hydrolysis of the imine product generates a disubstituted β-hydroxyaldehyde.
Enamines have been proposed as enolate donors in some aldol reactions. The intramolecular cyclization shown in equation 1 below may be induced by acid or base catalysis or by heating with a 2º-amine such as pyrrolidine. A few drops of acetic acid appear to enhance this catalysis, which probably takes place by the mechanism drawn in the colored box. If the 2º-amine and carboxylic acid functions are incorporated in the same molecule, as for example in the amino acid proline, exceptional catalytic action might be expected. This has been realized in a recent study reported by Alan B. Northrup and David W. C. MacMillan from Cal. Tech. As shown in equation 2, catalytic pyrrolidine effects the homo-condensation of propanal to anti-3-hydroxy-2-methylpentanal under mild conditions. Apparently β-hydroxyaldehydes resist enamine formation, since there is no further reaction of this product. In contrast, alkali metal hydroxides cause polymerization of this aldehyde. Of particular value in this reaction is its' high enantioselectivity. The previously described aldol reactions generate racemic mixtures of stereoisomers from achiral reactants. In this case, enantiomerically pure (S)-proline (the natural amino acid) produces anti-(2S,3S)-3-hydroxy-2-methylpentanal in 99% enantiomeric excess. As expected, (R)-proline catalyzes formation of the enantiomer.
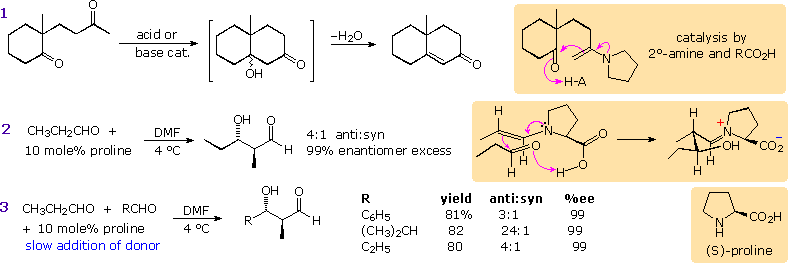

Since the rate of enamine formation from aldehydes is retarded by bulky substituents, cross condensations with 2-propanal are possible, provided this donor aldehyde is added slowly to the acceptor-catalyst mixture. Three examples are shown in equation 3. A mechanism for these stereoselective reactions is drawn in the colored box to the right of equation 2. Proline transfer from the iminium aldol species to a new aldehyde molecule may be assisted by the small amounts of water produced in the initial enamine formation.
This transformation is similar to the aldol-retroaldol processes catalyzed by a family of enzymes called aldolases. Lysine and aspartic acid functions have been identified in the active site of this enzyme. Configurations of the reactants and intermediates are not indicated, but these transformations are highly stereospecific. Antibodies that mimic this enzymatic catalysis have been prepared and used effectively in enantioselective synthesis.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


