
Barrelene
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 11-7-2018
11-7-2018
 1604
1604
Barrelene
Formulation of the Hückel rule prompted organic chemists to consider the possible aromaticity of many unusual unsaturated hydrocarbons. One such compound was the 6 π-electron bicyclic structure, now known as barrelene. Although the π-bonds in barrelene are not coplanar, it was believed that transannular overlap might still lead to aromatic stabilization.
A synthesis of barrelene (bicyclo[2.2.2]-2,5,7-octatriene) was accomplished nearly fifty years ago by H. Zimmerman (Wisconsin), using a double Hofmann elimination. As shown in the following diagram, the chemical behavior of this triene confirmed it was not aromatic in the accepted sense of this term. Bromine addition took place rapidly with transannular bond formation, in the same fashion as with norbornadiene (bicyclo[2.2.1]-2,5-heptadiene). Pyrolysis of barrelene gave the expected cycloreversion products benzene and acetylene.
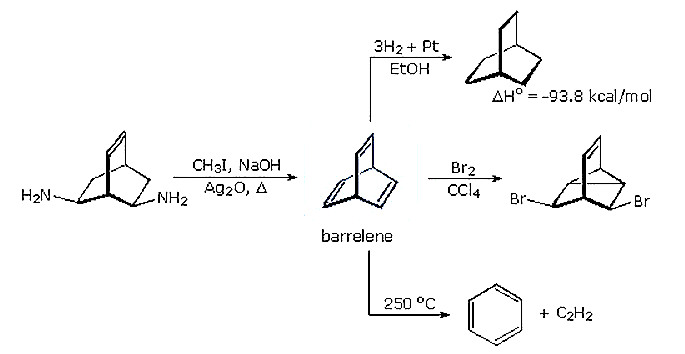
The heat of hydrogenation of barrelene reflects its thermodynamic stability. The value for cyclohexene is -28 kcal/mol, significantly less than one third of the barrelene number. Furthermore, the first double bond of barrelene is reduced with the release of 36.7 kcal/mol heat, indicating destabilization rather than stabilization.
The electronic spectrum of barrelene shows a π-electron interaction similar to that in related homoconjugated dienes. (λmax ≅220-230 nm).
An explanation for the lack of aromatic behavior in the case of barrelene may be found by comparing the orbital symmetry of the six component p-orbitals with those of benzene. Benzene is an annulene in which all six p-orbitals may be oriented with congruent overlapping phases. The cylindrical array of p-orbitals in barrelene cannot be so arranged, as shown in the diagram on the right. There will always be one region (a nodal plane) in which the transannular overlap is incongruent. By clicking on this diagram, a Jmol model of barrelene will be displayed in a separate window. This model may be moved about for viewing. The p-orbitals of the double bonds may also be displayed.
 الاكثر قراءة في كيمياء عامة
الاكثر قراءة في كيمياء عامة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


