


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-11-2019
Date: 19-9-2018
Date: 22-11-2019
|
Imine and Hydrazone Anions
Still another way of circumventing some of the undesired aspects of enolate anion chemistry is to replace the oxygen of an aldehyde or ketone substrate with a 1º-amino group, in other words, to convert the carbonyl function to an imine. Imine derivatives are relatively easy to prepare, starting with an aldehyde or ketone and a 1º-amine or hydrazine derivative. The resulting C=N function does not activate alpha-C-H groups as effectively as a carbonyl function, but very strong bases such as LDA, alkyl lithiums and Grignard reagents will convert imines to their enamide conjugate bases quantitatively. This general reaction is shown in the green shaded box below.
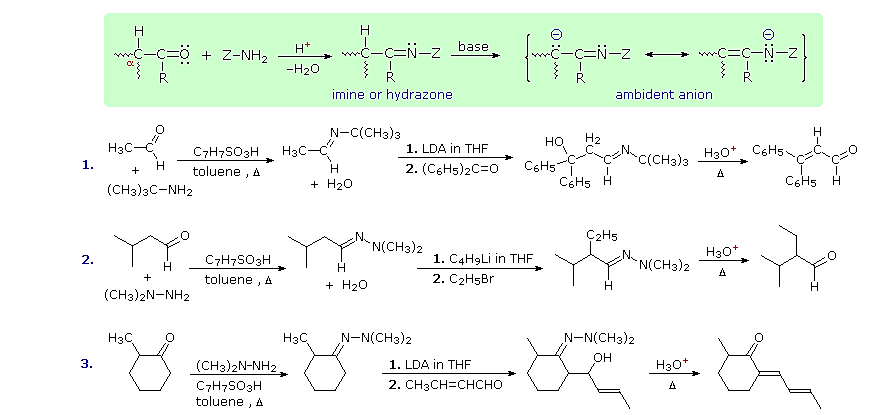
Three illustrations of the use of enamide bases in synthesis are displayed above. The first two examples use aldehyde derivatives, and if we were to attempt these reactions with the aldehyde enolate anion itself, aldol dimerization would result. The C=N function of imines is a poor acceptor of nucleophiles, so it does not assume such a role in aldol-like reactions. The third reaction is an aldol condensation in which a ketone serves as the donor. If cuprous salts are introduced before the unsaturated aldehyde is added to the enamide solution, conjugate addition takes place in preference to the 1,2-aldol addition.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|