

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Boiling Point Elevation
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 93
16-7-2017
2452
Boiling Point Elevation
Since the vapor pressure of the solvent is lowered by the presence of solutes, the temperature at which the vapor pressure equals atmospheric pressure (where the solution boils) is higher than for the pure solvent, as shown in Figure 1.1. Detailed calculations show that the increase in boiling point for a dilute solution is proportional to the total molal concentration of solutes:
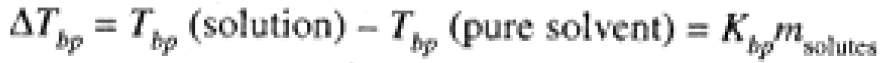 (1.1)
(1.1)
Kbp is the molal boiling-point constant, a property of the solvent and independent of the nature of the solutes; msolutes refers to the total concentration of independent solute particles whether they are neutral molecules or ions. Thus a 0.01 molal NaCl solution has a total solute concentration (Na+ and Cl-) of 0.02 mol/kg. Eq (1-1) is accurate for dilute solutions: For non-ionic solutes, this generally means less than 0.1 mol/kg; for ionic solutes, less than 0.01 mol/kg. For more concentrated solutions, the boiling point is increased but not precisely in proportion to the solute molality.
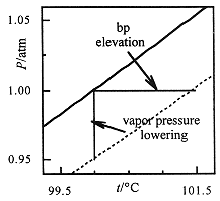
Figure 1.1. Liquid and vapor are at equilibrium along the vapor pressure curves shown for pure water (solid line) and an aqueous solution (dashed line). The vapor pressure is lower for the solution, in accord with Raoult's law, and thus the boiling point is increased (liquids boil at 1 atm).
A boiling point elevation measurement can be used to estimate the molar mass of a solute.
Example 1
3.75 g of a nonvolatile solute was dissolved in 95 g of acetone. The boiling point was 56.50°C compared with 55.95°C for pure acetone. If Kbp = 1.71 K kg/mol for acetone, what is the approximate molar mass of the solute? From Eq. (11-3), we have
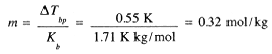
The molar mass thus is
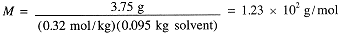
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















