
Variable oxidation states
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p539
الجزء والصفحة:
2th ed p539
 23-2-2017
23-2-2017
 1877
1877
Variable oxidation states
The occurrence of variable oxidation states and, often, the interconversion between them, is a characteristic of most d-block metals; exceptions are in groups 3 and 12 as Table 19.3 illustrates.
Table 19.3 Oxidation states of the d-block metals; the most stable states are marked in blue. Tabulation of zero oxidation states refers to their appearance in compounds of the metal.
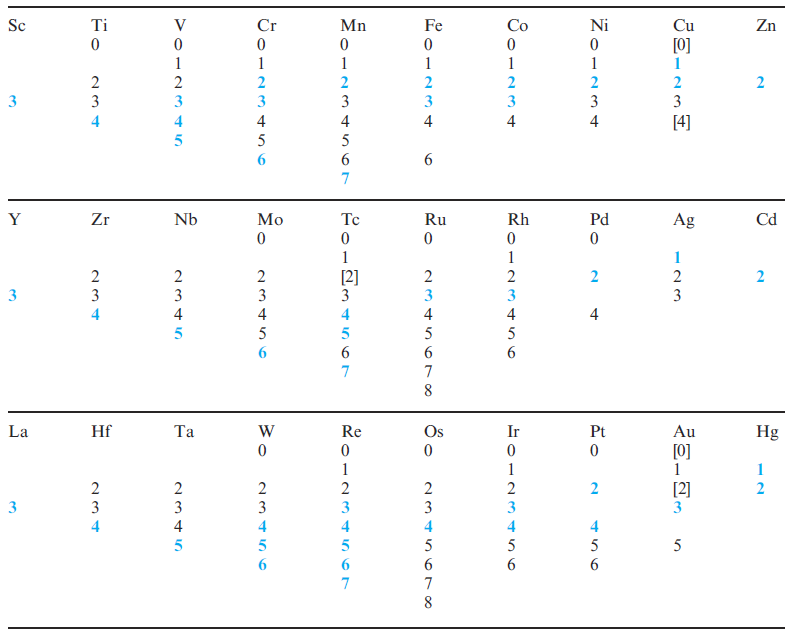
A comparison between the available oxidation states for a given metal and the electronic configurations listed in Table 1.3 is instructive. As expected, metals that display the greatest number of different oxidation states occur in or near the middle of a d-block row. Two cautionary notes (illustrated by d- and f -block metal compounds) should be made:
- The apparent oxidation state deduced from a molecular or empirical formula may be misleading, e.g. LaI2 is a metallic conductor and is best formulated as La3+(I-)2(e-), and MoCl2 contains metal cluster units with metal–metal bonds and is formally [Mo6Cl8]4+(Cl-)4. Indeed, metal–metal bond formation becomes more important for the heavier metals.
- There are many metal compounds in which it is impossible to assign oxidation states unambiguously, e.g. in the complexes [Ti(bpy)3]n- (n = 0, 1, 2), there is evidence that the negative charge is localized on the bpy ligands not the metal centres, and in nitrosyl complexes, the NO ligand may donate one or three electrons.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


