
OXIDATION BY CYTOCHROME P450S
 المؤلف:
James R Hanson
المؤلف:
James R Hanson
 المصدر:
Chemistry and Medicines
المصدر:
Chemistry and Medicines
 الجزء والصفحة:
p26
الجزء والصفحة:
p26
 18-1-2016
18-1-2016
 1307
1307
OXIDATION BY CYTOCHROME P450S
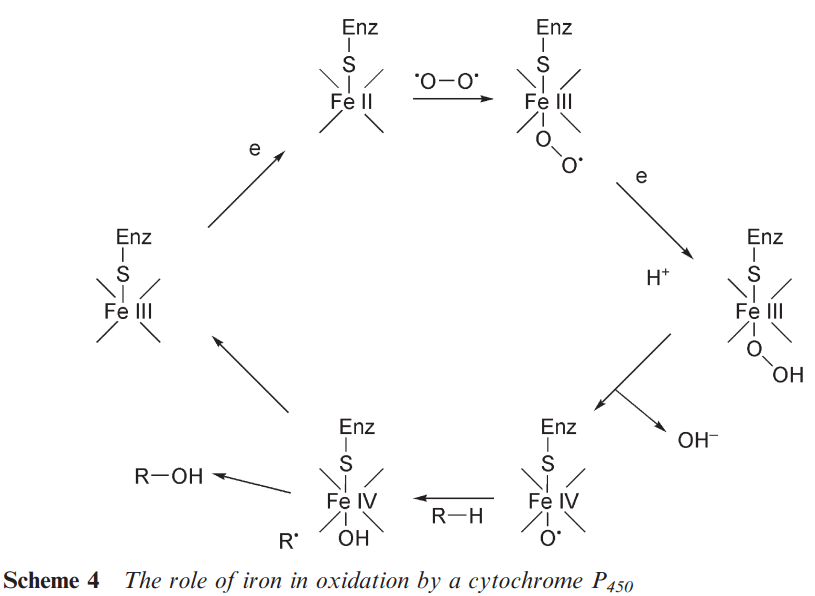
There are a variety of oxidative changes that may occur to a drug. Many of these involve the insertion of oxygen using a cytochrome P450-dependent enzyme. The cytochromes contain haem 2.5 as the co-enzyme. The oxygen is bound by the central iron and delivered to the substrate by a series of redox changes (see Scheme 4). There are a series of different cytochromes, CYP1A1, CYP1A2, CYP2A6, CYP2C9, CYP2C10, CYP2C18, CYP3A, CYP4A4 etc. in the liver. The cytochrome CYP3A4 is responsible for the oxidation of many drugs. Each of these, while containing the haem co-enzyme, has a range of different substrate specificities, hence the name ‘mixed function oxidases’. The relative proportion of these enzymes can differ between individuals and thus there are variations in the metabolism of drugs. Some individuals may lack a particular cytochrome. Consequently, toxic metabolites may only become apparent after a drug has been in use with a large number of patients.
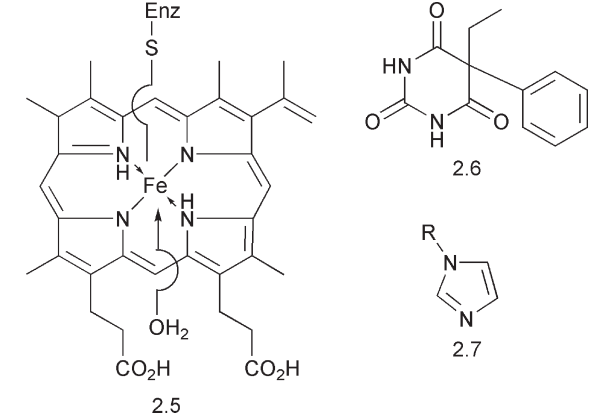
Some cytochrome P450s in the liver are induced by specific drugs, e.g. phenobarbital 2.6 while others may be inhibited by drugs, e.g. by azoles 2.7, which complex with iron. For example, the anti-ulcer drug, cimetidine, which contains an imidazole ring, binds to the iron of cytochrome P450s. This alters the metabolism and hence biological activity of other drugs. Ethanol and a constituent of grapefruit juice, nootkatone, also have an effect on cytochrome P450s. This can lead to an altered pattern of drug metabolism and to drug:drug interactions.
 الاكثر قراءة في الكيمياء الطبية والدوائية
الاكثر قراءة في الكيمياء الطبية والدوائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


