
The distillation of mixtures
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص181-182
الجزء والصفحة:
ص181-182
 2025-11-16
2025-11-16
 27
27
The distillation of mixtures
The region between the lines in Fig. 6.14 is a two-phase region where F′=1. As usual, the prime indicates that one degree of freedom has been discarded; in this case, the pressure is being kept fixed, and hence at a given temperature the compositions of the phases in equilibrium are fixed. The regions outside the phase lines correspond to a single phase, so F′=2, and the temperature and composition are both independently variable.
Consider what happens when a liquid of composition a1 is heated. It boils when the temperature reaches T2. Then the liquid has composition a2 (the same as a1) and the vapour (which is present only as a trace) has composition a2 ′. The vapour is richer in the more volatile component A (the component with the lower boiling point). From the location of a2, we can state the vapour’s composition at the boiling point, and from the location of the tie line joining a2 and a2 ′ we can read off the boiling tempera ture (T2) of the original liquid mixture.
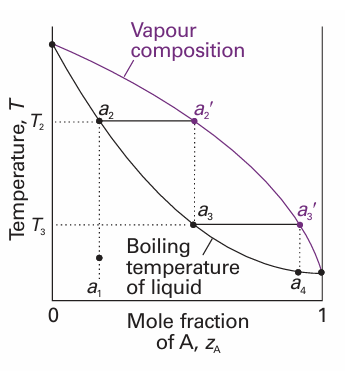
Fig. 6.14 The temperature–composition diagram corresponding to an ideal mixture with the component A more volatile than component B. Successive boilings and condensations of a liquid originally of composition a1 lead to a condensate that is pure A. The separation technique is called fractional distillation.
In a simple distillation, the vapour is withdrawn and condensed. This technique is used to separate a volatile liquid from a non-volatile solute or solid. In fractional dis tillation, the boiling and condensation cycle is repeated successively. This technique is used to separate volatile liquids. We can follow the changes that occur by seeing what happens when the first condensate of composition a3 is reheated. The phase diagram shows that this mixture boils at T3 and yields a vapour of composition a3 ′, which is even richer in the more volatile component. That vapour is drawn off, and the first drop condenses to a liquid of composition a4. The cycle can then be repeated until in due course almost pure A is obtained. The efficiency of a fractionating column is expressed in terms of the number of theoretical plates, the number of effective vaporization and condensation steps that are required to achieve a condensate of given composition from a given distillate. Thus, to achieve the degree of separation shown in Fig. 6.15a, the fractionating column must correspond to three theoretical plates. To achieve the same separation for the system shown in Fig. 6.15b, in which the components have more similar partial pressures, the fractionating column must be designed to correspond to five theoretical plates.
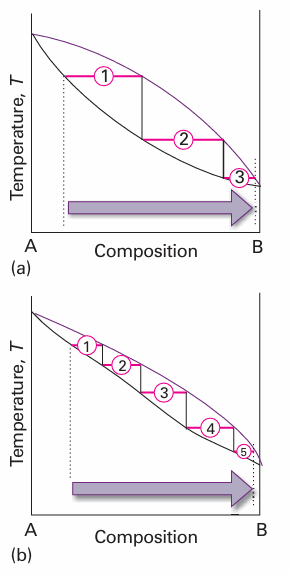
Fig. 6.15 The number of theoretical plates is the number of steps needed to bring about a specified degree of separation of two components in a mixture. The two systems shown correspond to (a) 3, (b) 5 theoretical plates.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


