
Oxidases
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص752-754
الجزء والصفحة:
ص752-754
 2025-10-25
2025-10-25
 286
286
Oxidases
Key points: Oxidases are enzymes that catalyse the reduction of O2 to water or hydrogen peroxide without incorporation of O atoms into the oxidizable substrate; they include cytochrome c oxidase, the enzyme that is a basis for all higher life forms. Cytochrome c oxidase is a membrane-bound enzyme that catalyses the four-electron re duction of O2 to water, using cytochrome c as the electron donor. The potential difference between the two half-cell reactions is over 0.5 V but this value does not reflect the true thermodynamics because the actual reaction catalysed by cytochrome c oxidase is:
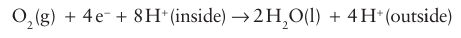
This reaction includes four H that are not consumed chemically but are ‘pumped’ across the membrane against a concentration gradient. Such an enzyme is called an electrogenic ion pump (or proton pump). In eukaryotes, cytochrome oxidase is located in the inner membrane of mitochondria and has many subunits (Fig. 27.35), although a simpler enzyme is produced by some bacteria. It contains three Cu atoms and two haem-Fe atoms, as well as a Mg atom and a Zn atom. The Cu and Fe atoms are arranged in three main sites. The active site for O2 reduction consists of a myoglobin-like Fe-porphyrin (haem-a3) that is situated close to a ‘semi-haemocyanin-like’ Cu (known as CuB) coordinated by three histidine ligands (35). One of the histidine imidazole ligands to the Cu is modified by for mation of a covalent bond to an adjacent tyrosine. Electrons are supplied to the dinuclear site by a second Fe porphyrin (haem-a) that is six-coordinate, as expected for an electron transfer centre. These centres are located in subunit 1. Subunit 2 contains the dinuclear CuA centre that was described in Section 27.8, which is believed to be the immediate acceptor of the electron arriving from cytochrome c. The electron transfer sequence is therefore
Cytochrome c → CuA → A haem a → binuclear site
The enzyme contains two proton-transfer channels, one of which is used to supply the protons needed for H2O production while the other is used for protons that are being pumped across the membrane. Figure 27.36 shows the proposed catalytic cycle.
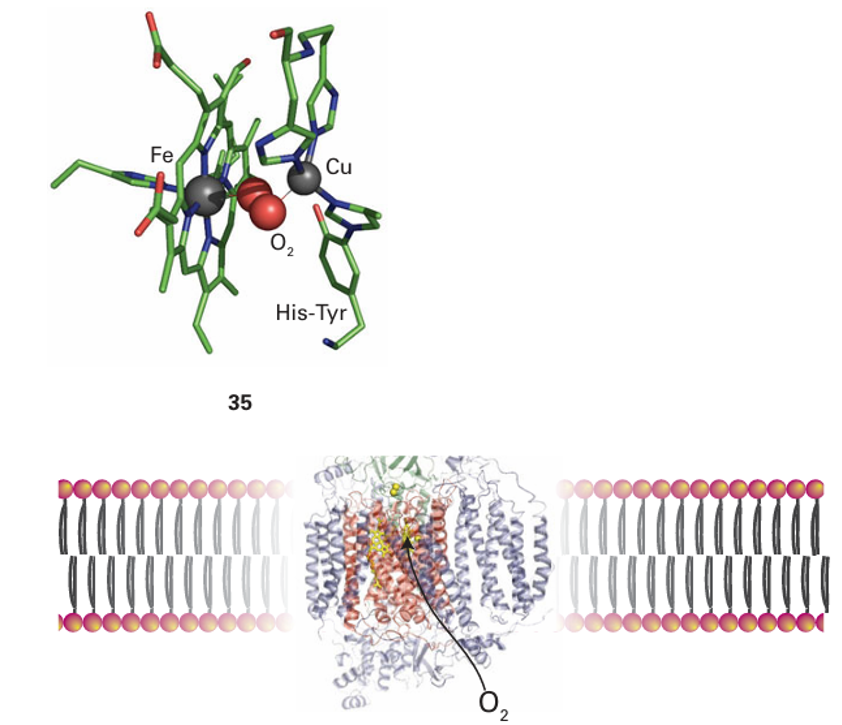
Figure 27.35 The structure of cytochrome c oxidase as it occurs in the membrane, showing the locations of the redox centres and the sites for reaction with O2 and cytochrome c. See (33) for a more detailed view of the active site.
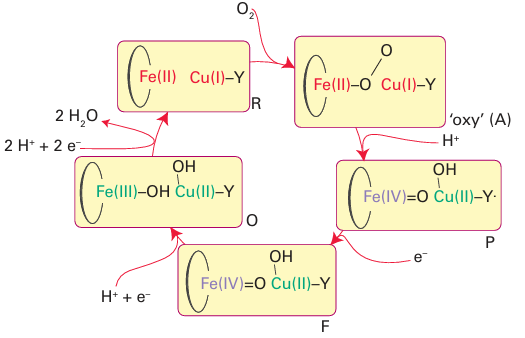
Figure 27.36 The catalytic cycle of cytochrome c oxidase. The intermediates are labelled according to current convention. Electrons are provided from the other haem and CuA. During the cycle, an additional four H are pumped across the membrane.
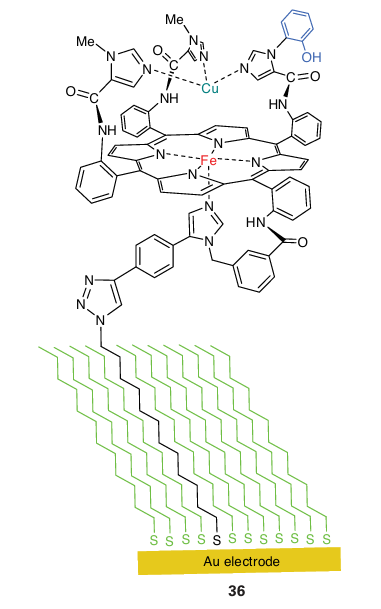
Starting from the state in which the active site is Fe(II) Cu(I), O2 binds to give an intermediate (oxy) that resembles oxymyoglobin. However, unlike oxymyoglobin, this intermediate takes up the other electron that is immediately available, producing a peroxy species that quickly breaks down to give an intermediate known as P. Species P has been trapped and studied by optical and EPR spectroscopy, which show that it contains Fe(IV) and an organic radical that may be located on the unusual His Tyr pair (Y.). The oxido-Fe(IV) (ferryl) group is formed by heterolytic cleavage of O2 , producing a water molecule. The role of a cation radical is again noted: without this radical, the Fe would have to be assigned as Fe(V). It is vital that intermediates such as peroxide are not released during conversion of O2 to water. Studies with an elaborate model complex (36) which can be attached to an electrode show that the presence of the phenol is crucial because it allows all four electrons necessary for the reduction of the O2 to be provided rapidly without relying on long-range electron transfer, which is slow through the long-chain aliphatic linker. If the phenolic OH group is replaced by -OCH3, hydrogen peroxide is released during O2 reduction because the methoxy derivative is unable to form an oxidized radical. The blue Cu oxidases contain a blue Cu centre that removes an electron from a substrate and passes it to a trinuclear Cu site that catalyses the reduction of O2 to H2 O. Two examples, ascorbate oxidase and a larger class known as laccases, are well characterized, whereas another protein, ceruloplasmin, occurs in mammalian tissue and is the least well understood. Ascorbate oxidase occurs in the skins of fruit such as squash. Its role may be twofold: to protect the flesh of the fruit from O2 and to oxidize phenolic substrates to in termediates that will form the skin of the fruit. Laccases are widely distributed, particularly
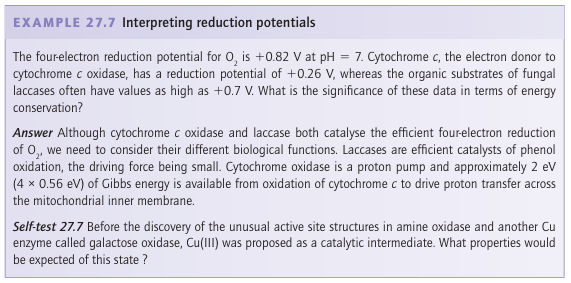
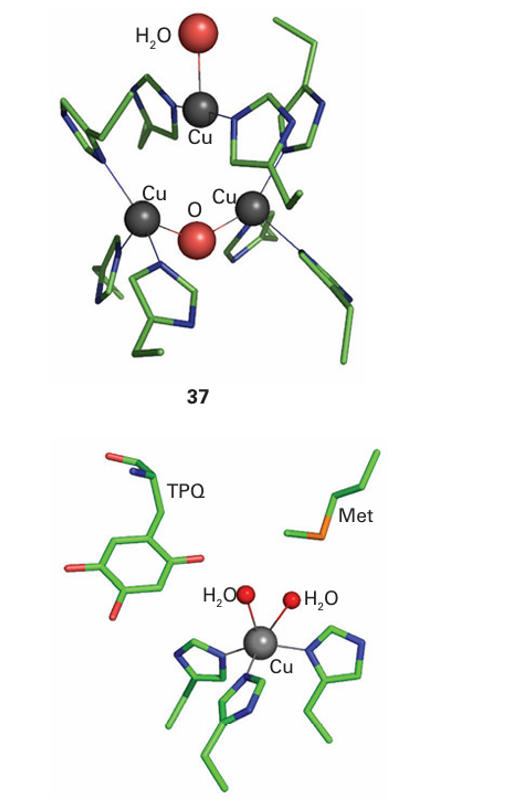
in plants and fungi, from which they are secreted to catalyse the oxidation of phenolic substrates. The active site at which O2 is reduced (37) is well buried. It contains a pair of Cu atoms linked in the oxidized form by a bridging O atom, with a third Cu atom situated very close by, completing an almost triangular arrangement. Amine oxidases catalyse the oxidation of amines to aldehydes by using just a single Cu atom that shuttles between Cu(II) and Cu(I), yet the enzyme carries out a two-electron reduction of O2 , producing a molecule of H2O2 . The problem is overcome because, like cytochrome c peroxidase and cytochrome c oxidase, amine oxidases have an additional oxidizing source located near to the metal, in this case a special cofactor called topaquinone (TPQ), which is formed by post-translational oxidation of tyrosine (38).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


