
Magnesium enzymes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
749
الجزء والصفحة:
749
 2025-10-25
2025-10-25
 300
300
Magnesium enzymes
Key points: The major direct catalytic function of magnesium is as the catalytic centre of ribulose bisphosphate carboxylase. The Mg2+ cation confers less polarization of coordinated ligands than Zn2+ (we often refer to Mg2+ as being a ‘weaker acid’ than Zn2); however, compared to Zn it is much more mobile and cells contain high concentrations of uncomplexed Mg2+ ions. Its major role in enzyme catalysis is as the Mg–ATP complex (1), which is the substrate in kinases, the en zymes that transfer phosphate groups thereby activating the target compound or causing it to change its conformation. Kinases are controlled by calmodulin (Section 27.4) and other proteins, so they are part of the signalling mechanism in higher organisms. An important example of an Mg enzyme in which Mg acts separately from ATP is ribulose 1,5-bisphosphate carboxylase, commonly known as ‘rubisco’. This enzyme, the most abundant in the biosphere, is responsible for the production of biomass by oxy genic photosynthetic organisms and removal of CO2 from the atmosphere (to the extent, globally, of over 1011 t of CO2 per year). Rubisco is an enzyme of the Calvin cycle, the stages of photosynthesis that can occur in the dark, in which it catalyses the incorporation of CO2 into a molecule of ribulose 1,5-bisphosphate (Fig. 27.31). The Mg2+ ion is octahedrally coordinated by carboxylate groups from glutamate and aspartate residues, three coordinated H2O molecules, and a carbamate derived from a lysine residue. The carbamate is formed by a reaction between CO2 and the side-chain –NH2, in an activation process that is necessary for Mg2 to bind. In the catalytic cycle, the binding of ribulose 1,5-bisphosphate displaces two H2O molecules, and proton abstraction assisted by the carbamate results in a coordinated enolate. This intermediate reacts with CO2, forming a new C–C bond, then the product is cleaved to yield two new three-carbon species and the cycle continues. The reactive enolate will also react with O2, in which case the result is an oxidative degradation of substrate: for this reason, the enzyme is often called ribulose 1,5-bisphosphate carboxylase-oxygenase. We note the difference from Zn, which would favour ligation by softer ligands and a lower coordination number. Rubisco requires a metal ion that combines good Lewis acidity with weak binding and high abundance.
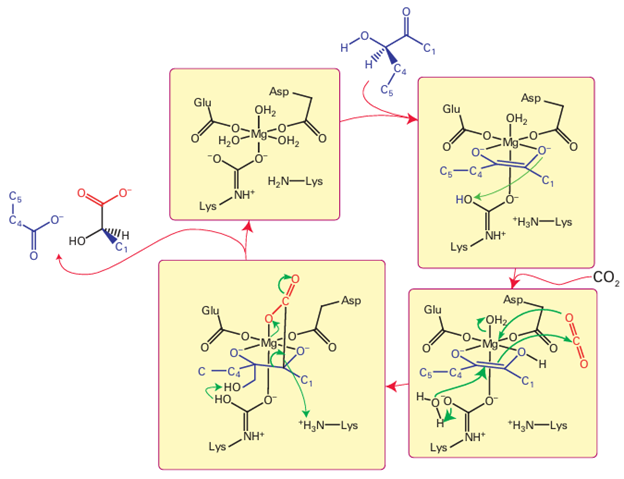
Figure 27.31 Mechanism of action of ribulose 1,5-bisphosphate carboxylase, the enzyme responsible for removing CO2 from the atmosphere and ‘fixing’ it in organic molecules in plants.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


