
General considerations
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص741-742
الجزء والصفحة:
ص741-742
 2025-10-23
2025-10-23
 320
320
General considerations
Key points: Electron flow along electron-transport chains is coupled to chemical processes such as ion (particularly H) transfer; the simplest electron-transfer centres have evolved to optimize fast electron transfer. In organisms, electrons are abstracted from food (fuel) and flow to an oxidant, down the potential gradient formed by the sequence of acceptors and donors known as a respiratory chain (Fig. 27.22).7 Apart from flavins and quinones, which are redox-active organic cofactors, these acceptors and donors are metal-containing electron transfer (ET) centres, which fall into three main classes, namely FeS clusters, cytochromes, and Cu sites. These enzymes are generally bound in a membrane, across which the energy from ET is used to sustain a transmembrane proton gradient: this is the basis of the chemiosmotic theory. The counterflow of H, through a rotating enzyme known as ATP synthase, drives the phosphorylation of ADP to ATP. Many membrane-bound redox enzymes are electrogenic proton pumps, which means they directly couple long-range ET to proton transfer through specific internal channels. We shall examine the properties of the three main types of ET centre. The same rules concerning outer-sphere electron-transfer that were discussed in Section 21.12 apply to metal centres in proteins, and we should note that organisms have optimized the structures and properties of these centres to achieve efficient long-range electron transfer. In the following discussion it will be useful to keep in mind that reduction potentials de pend on several factors (Section 5.10). Besides ionization energy and ligand environment (strong donors stabilize high oxidation states and lower the reduction potential; weak donors, π acceptors, and protons stabilize low oxidation states and raise the reduction potential), an active site in a protein is also influenced by the relative permittivity (which
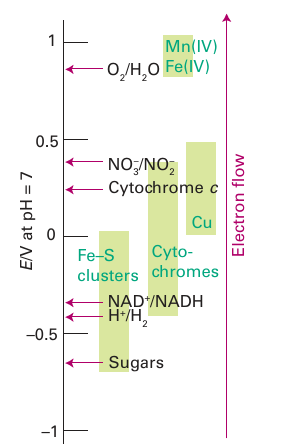
Figure 27.21 The ‘redox spectrum’ of life
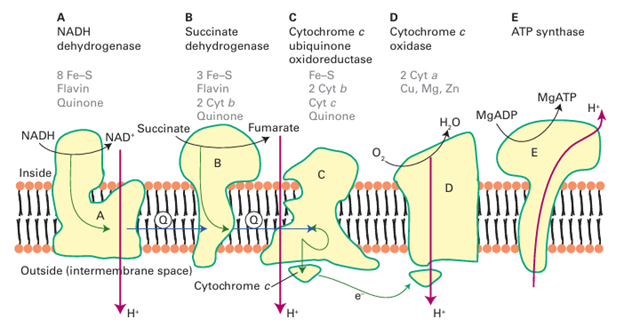
Figure 27.22 The mitochondrial respiratory electron transfer (ET) chain consists of several metalloenzyme molecules that use the energy of electron transport to transport protons across a membrane. The proton gradient is used to drive ATP synthesis. stabilizes centres with low overall charge), the presence of neighbouring charges, including those provided by other bound metal ions, and the availability of hydrogen-bonding interactions that will also stabilize reduced states. When we consider the kinetics of electron transfer, it will similarly be useful to keep in mind that ‘efficiency’ means that electron transfer is fast even when the reaction Gibbs energy is low and therefore that the reorganization energy of Marcus theory (Section 21.12) is low. This requirement is met by providing a ligand environment that does not alter significantly when an electron is added and by burying the site so that water molecules are excluded. Intersite distances are generally less than 1.4 nm in order to facilitate electron tunnelling, although it is still debated whether electron transfer in proteins depends mainly on distance alone or whether the protein can provide special pathways.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


