
Sulfur dioxide oxidation
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
710
الجزء والصفحة:
710
 2025-10-19
2025-10-19
 306
306
Sulfur dioxide oxidation
Key point: The most widely used catalyst for the oxidation of SO2 to SO3 is molten potassium vanadate supported on a high-surface-area silica. The oxidation of SO2 to SO3 is a key step in the production of sulfuric acid (Section 16.13). The reaction of sulfur with oxygen to produce SO3 gas is exergonic (∆rGO 371 kJ mol 1) but very slow, and the principal product of combustion is SO2:
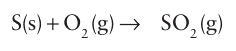
The combustion is followed by the catalytic oxidation of SO2:
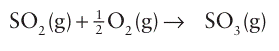
This step is also exothermic and thus, as with ammonia synthesis, has a less favourable equilibrium constant at elevated temperatures. The process is therefore generally run in stages. In the first stage, the combustion of sulfur raises the temperature to about 600ºC, but by cooling and pressurizing before the catalytic stage the equilibrium is driven to the right and high conversion of SO2 to SO3 is achieved. Several quite different catalytic systems have been used to catalyse the combination of SO2 with O2. The most widely used catalyst at present is potassium or caesium vanadate molten salt covering a high-surface-area silica. The current view of the mechanism of the re action is that the rate-determining step is the oxidation of V(IV) to V(V) by O2 (Fig. 26.21). In the melt, the vanadium and oxide ions are part of a polyvanadate complex (Section 19.8), but little is known about the evolution of the oxo species.
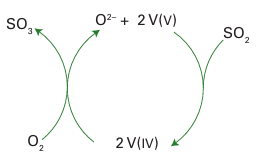
Figure 26.21 Cycle showing the key elements involved in the oxidation of SO2 by V(V) compounds.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


