
Surface acidic and basic sites
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص705-706
الجزء والصفحة:
ص705-706
 2025-10-18
2025-10-18
 271
271
Surface acidic and basic sites
Key point: Surface acids and bases are highly active for catalytic reactions such as the dehydration of alcohols and isomerization of alkenes. When exposed to atmospheric moisture, the surface of γ-alumina is covered with adsorbed water molecules. Dehydration at 100 to 150°C leads to the desorption of water, but sur face OH groups remain and act as weak Brønsted acids:
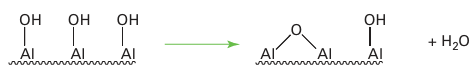
At even higher temperatures, adjacent OH groups condense to liberate more H2O and generate exposed Al3 Lewis acid sites as well as O2 Lewis base sites (11). The rigidity of the surface permits the coexistence of these strong Lewis acid and base sites, which would otherwise immediately combine to form Lewis acid–base complexes. Surface acids and bases are highly active for catalytic reactions such as the dehydration of alcohols and isomerization of alkenes. Similar Brønsted and Lewis acid sites exist on the interior of certain zeolites. Different oxides and their mixtures show variations in surface acidity; thus a SiO2 /TiO2 mixture is more acidic than SiO2 /Al2O3, and promotes different catalytic reactions.

Highly active acidic and basic surfaces act as useful substrates for depositing other catalytic centres, particularly metal particles. Treatment of γ-alumina with H2PtCl6 followed by heating in a reducing environment produces Pt particles of dimensions 1–50 nm distriuted over the alumina surface.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


