
Asymmetric oxidations
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
701
الجزء والصفحة:
701
 2025-10-18
2025-10-18
 310
310
Asymmetric oxidations
Key point: Appropriate chiral ligands can be used in conjunction with d-metal catalysts to induce chirality into oxidation products of organic substrates. In addition to catalysing reductions, d-metal complexes are also active in oxidations. For example, in the Sharpless epoxidation, prop-2-en-1-ol (allyl alcohol) or a derivative is oxidized with tert-butylhydroperoxide, in the presence of a Ti catalyst with diethyl tartrate as a chiral ligand, producing an epoxide:
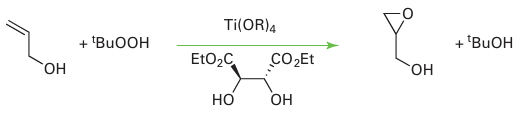
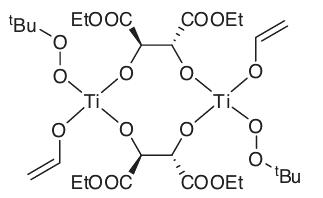
The reaction is thought to go through a transition state in which both the peroxide and the allyl alcohol are coordinated to a Ti atom through their O atoms. Each Ti atom is known to have one diethyl tartrate attached to it, and the chiral environment that the diethyl tartrate produces around the Ti atom is sufficient to differentiate the two prochiral faces of the allyl alcohol. Additional experimental evidence points to a dimeric intermediate (8). Enantiomeric excesses of greater than 98 per cent have been reported for Sharpless epoxidations.
A Jacobsen oxidation is a reaction in which the catalyst is a Mn complex of the mixed 2xN+2xO donor ligand known as salen (Fig. 26.10). Hypochlorite ions (ClO-) are used to oxidize the Mn (III) complex to a Mn(V) oxide, which is then able to deliver its O atoms to an alkene to generate an epoxide. This oxidation has been used with a wide variety of substrates and routinely delivers enantiomeric excesses greater than 95 per cent. The mechanism of the reaction has not been defined precisely, but proposals include the existence of a dimeric form of the catalyst or a radical oxygen transfer step.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


