
Glass composition, production, and application
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص624-625
الجزء والصفحة:
ص624-625
 2025-10-11
2025-10-11
 240
240
Glass composition, production, and application
Key points: Low-valence metal oxides, such as Na2O and CaO, are often added to silica to reduce its softening temperature by disrupting the silicon–oxygen framework. Other cations may be incorporated into glasses, giving applications as diverse as lasers and nuclear waste containment. Although vitreous silica is a strong glass that can withstand rapid cooling or heating without cracking, it has a high glass transition temperature and therefore must be worked at in conveniently high temperatures. Therefore, a modifier, such as Na2O or CaO, is commonly added to SiO2. A modifier disrupts some of the Si-O-Si linkages and replaces them with terminal Si O links that associate with the cation (Fig. 24.34). The consequent partial disruption of the Si O network leads to glasses that have lower softening points. The common glass used in bottles and windows is called ‘sodalime glass’ and contains Na2O and CaO as modifiers. When B2O3 is used as a modifier, the resulting ‘borosilicate glasses’ have lower thermal expansion coefficients than sodalime glass and are less likely to crack when heated. Borosilicate glass (such as Pyrex®) is therefore widely used for ovenware and laboratory glassware. Glass formation is a property of many oxides, and practical glasses have been made from sulfides, fluorides, and other anionic constituents. Some of the best glass formers are the oxides of elements near silicon in the periodic table (B2O3, GeO2, and P2O5), but the solubility in water of most borate and phosphate glasses and the high cost of germanium limit their usefulness.
The development of transparent crystalline and vitreous materials for light transmission and processing has led to a revolution in signal transmission. For example, optical fibres are currently being produced with a composition gradient from the interior to the surface. This composition gradient modifies the refractive index and thereby decreases light loss. Fluoride glasses are also being investigated as possible substitutes for oxide glasses because oxide glasses contain small numbers of OH groups, which absorb near-infrared radiation and attenuate the signal. Doping lanthanoid ions into the glass fibres produces materials that can be used to amplify the signals by using lasing effects. Optical circuit elements are being developed that may eventually replace all the components in an electronic integrated circuit and lead to very fast optical computers. As modifier cations effectively become trapped in a glass, which is chemically inert and thermodynamically very stable with respect to transformation to a soluble, crystalline phase, such glassy materials offer a potential method for containing and storing nuclear waste. Thus, vitrification of metal oxides containing a radioactive species with glass-forming oxides produces a stable glass that can be stored for long periods, allowing the radionu-cleide to decay (Section 23.1). Examples of such materials are borosilicate glasses and combinations of such glasses with crystalline oxide phases (‘Synroc’). The incorporation of functional inorganic compounds into glasses has led to the development of so-called ‘smart glasses’ that have switchable properties, such as electro chromism, the ability to change colour or light transmission properties in response to application of a potential difference, and reversible photochromism, the ability to change colour under certain light conditions. An electrochromic glass generally consists of a glass coated with colourless, tungsten trioxide, WO3, or a sandwich-like layer structure consist ing of these two components. When a potential difference is applied to the WO3 layer, cations are inserted and the W is partially reduced to form Mx W(VI,V)O3, which is dark blue. The coating maintains its dark colour until the potential difference is reversed, when the glass becomes colourless. Application of electrochromic glasses include privacy glass, auto-dimming rear view mirrors in vehicles, and aircraft windows. A similar coating is used in self-cleaning glasses where a metal oxide coating on the glass acts as a photocatalyst for the breakdown of organic dirt on their surfaces. A photochromic glass incorporates a small amount of a colourless silver halide, normally AgCl, within the glass. When exposed to UV radiation, such as that in sunlight, the AgCl dissociates to form small clusters of Ag atoms, which absorb light across the visible region of the spectrum, imparting a grey colour to the glass. When the UV radiation is removed, the AgCl reforms and the glass returns to its optically transparent state. Photochromic glasses are widely used in the lenses of spectacles and sunglasses.
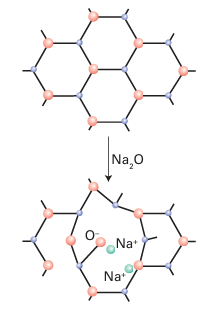
Figure 24.34 The role of a modifier is to introduce O2- ions and cations that disrupt the lattice.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


