
Solid anionic electrolytes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص609-610
الجزء والصفحة:
ص609-610
 2025-10-08
2025-10-08
 298
298
Solid anionic electrolytes
Key point: Anion mobility can occur at high temperatures in certain structures that contain high levels of anion vacancies. Michael Faraday reported in 1834 that red-hot solid PbF2 is a good conductor of electric ity. Much later it was recognized that the conductivity arises from the mobility of F ions through the solid. The property of anion conductivity is shared by other crystals having the fluorite structure. Ion transport in these solids is thought to be by an interstitial mechanism in which an F ion first migrates from its normal position into an interstitial site (a Frenkel-type defect, Section 3.16) and then moves to a vacant F site. Structures that have large numbers of vacant sites generally show the highest ionic conductivities because they provide a path for ion motion (although at very high levels of defects, clustering of the defects or the vacancies can lower the conductivity). These vacancies, which are equivalent to extrinsic defects, can be introduced in fairly high numbers into many simple oxides and fluorides by doping with appropriately chosen metal ions in different oxidation states. Zirconia, ZrO2, at high temperature has a fluorite structure, but on cooling the pure material to room temperature it distorts to a monoclinic polymorph. The cubic fluorite structure may be stabilized at room temperature by replacing some Zr4+ with other ions, such as the similarly sized Ca2 and Y3 ions. Doping with these ions of lower oxidation number results in the introduction of vacancies on the anion sites to preserve the charge neutrality of the material and produces, for example, YxZr1-x O2-x/2, the material mentioned previously as ‘yttrium stabilized zirconia’ (YSZ). This material has completely occupied cation sites in the fluorite structure but high levels of anion vacancies, with 00.15 Zr0.85 O1.85 is 5 S cm-1 at 1000°C;1 note that this conductivity is much lower than typical solid-state cation conductivities—even at these very high temperatures—due to the large anion size. The high oxide-ion conductivity of calcium-oxide doped zirconia is exploited in a solid-state electrochemical sensor for measuring the partial pressure of oxygen in automobile exhaust systems (Fig. 24.9).2 The platinum electrodes in this cell adsorb O atoms and, if the partial pressures of oxygen are different between the sample and reference side, there is a thermodynamic tendency for oxygen to migrate through the electrolyte as the O2 ion. The thermodynamically favoured processes are:
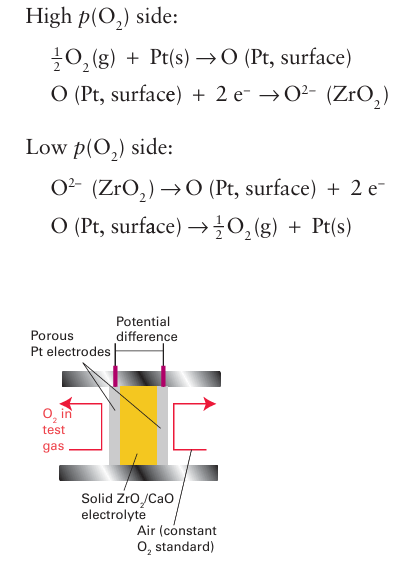
Figure 24.9 An oxygen sensor based on the solid electrolyte Zr1-x CaxO2-x .
The cell potential is related to the two oxygen partial pressures (p1 and p2) by the Nernst equation (Section 5.5), for the half-cell reaction O2+4e- → 2O2- , which occurs at both electrodes.
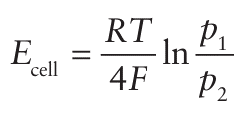
so a simple measurement of the potential difference provides a measure of the oxygen partial pressure in the exhaust gases. A brief illustration. According to this equation, the potential difference produced by an oxygen sensor operating at 1000 K in an exhaust system, with air on one side (p(O2) =0.2 atm) and a burnt fuel/air mixture (p(O2) =0.001 atm) on the other, is about 0.1 V. Behaviour similar to that of YSZ is encountered for other compounds that adopt the fluorite structure, such as PbF2 as discovered by Faraday. As noted previously, the anionic conductivities remain low even at high temperatures, so many other complex metal oxides are currently being investigated with the aim of achieving high mobilities at low tempera tures. Some compounds that show promising behaviour include La2 Mo2O9, barium indate (Ba2In2O5), BIMEVOX (a d-metal doped bismuth vanadium oxide), the apatite structure of La9.33Si6 O26, and strontium- and magnesium-doped lanthanum gallate (Sr, Mg-doped LaGaO3, or LSGM). As well as uses in sensor devices, materials possessing oxide- and proton-ion conductivity are important in a number of fuel cell types .
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


