
Solid cationic electrolytes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص607-609
الجزء والصفحة:
ص607-609
 2025-10-08
2025-10-08
 283
283
Solid cationic electrolytes
Key points: Solid inorganic electrolytes often have a low-temperature form in which the ions are or dered on a subset of sites in the structure; at higher temperatures the ions become disordered over the sites and the ionic conductivity increases.
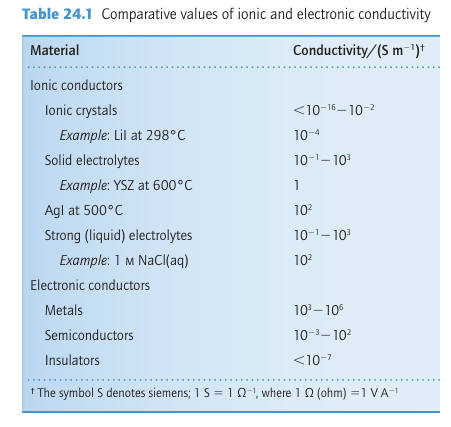
Below 50°C, Ag2 HgI4 has an ordered crystal structure in which Ag and Hg ions are tetrahedrally coordinated by I ions and there are unoccupied tetrahedral holes (Fig. 24.5a). At this temperature its ionic conductivity is low. Above 50°C, however, the Ag+ and Hg+2 ions are randomly distributed over the tetrahedral sites (Fig. 24.5b) and as a result there are many more sites that Ag+ ions can occupy within the structure than there are Ag ions present. At this temperature the material is a good ionic conductor, largely on account of the mobility of the Ag ions between the different sites available for it. The close-packed array of polarizable I- ions is easily deformed and results in a low activation energy for the migration of an Ag ion from one ion site to the next. There are many related solid electrolytes having similar structures containing soft anions, such as AgI and RbAg4I5, both of which have highly mobile Ag+ ions so that the conductivity of RbAg4I5 at room temperature is greater than that of aqueous sodium chloride.
Sodium-alumina is an example of a mechanically hard material that is a good ionic conductor. In this case, the rigid and dense Al2O3 slabs are bridged by a sparse array of O2- ions (Fig. 24.6). The plane containing these bridging ions also contains Na ions, which can move from site to site because there are no major bottlenecks to hinder their motion. Many similar rigid materials having planes or channels through which ions can move are known; they are called framework electrolytes. Another closely related material, sodium -alumina, has even less restricted motion of ions than -alumina, and it has been found possible to substitute doubly charged cations such as Mg+2 or Ni+2 for Na+. Even the large lanthanoid cation Eu2 can be introduced into -alumina, although the diffusion of such ions is slower than that of their smaller counterparts. The material NASICON, mentioned earlier, is a nonstoichiometric, solid-solution system with a framework constructed from ZrO6 octahedra and PO4 tetrahedra, corresponding to the parent phase of composition NaZr2P3O12 (Fig. 24.7). A solid solution can be obtained by partially replacing P by Si to give Na1+x Zr2P3-x SixO12 with an increase in the number of Na ions for charge balance. In this material, the full set of possible Na sites is only partially filled and these sites lie with in a three-dimensional network of channels that allow rapid migration of the remaining Na ions. Other classes of materials currently being investigated as fast cation conductors include Li4GeO4 doped with V on the Ge sites (Li4 x (Ge1-x Vx) O4, a lithium-ion conductor with vacancies on the Li ion sub-lattice), the perovskite La0.6 Li0.2TiO3, and sodium yttrium silicate, Na5YSi4O12 (a sodium-ion conductor).
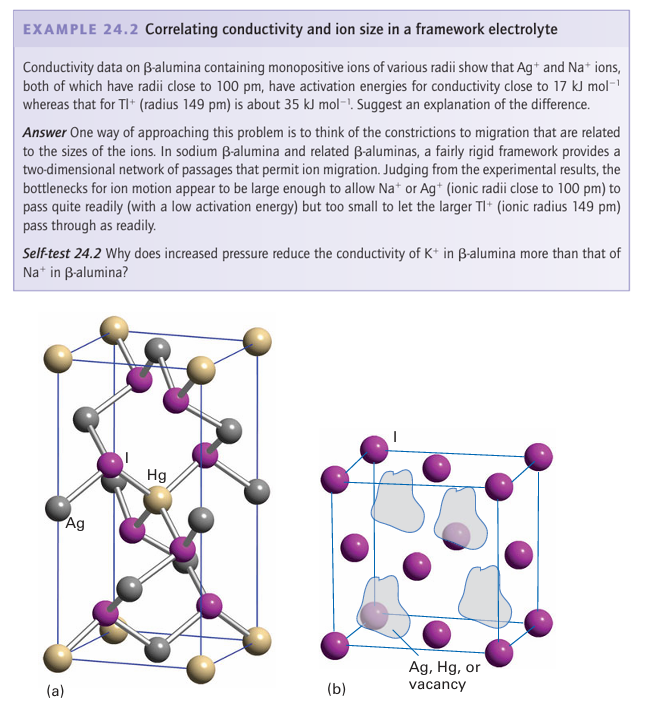
Figure 24.5 (a) Low-temperature ordered structure of Ag2HgI4. (b) High-temperature disordered structure showing the cation disorder. Ag2HgI4 is an Ag ion conductor in the high temperature form. Because the reactivity of a bulk material is related to the presence of crystal defects and to the processes of atom and ion diffusion, by modelling an ion diffusion process information can be obtained on both ion conduction and the reaction mechanisms of a solid. Such a study has been undertaken on Li3N with the aim of determining the energy barriers for Li+ ions moving through the structure. Comparison of the values for the various barriers between all the different possible sites that Li+ could occupy as it migrates through the solid in turn allows proposals to be made for the conduction pathway and a value for ionic conductivity to be calculated. Diffusion of Li+ ions in the Li2N plane (Fig. 24.8) was found to have a much lower energy barrier than for motion perpendicular to the plane. The effect of replacing the Li+ ions between the layers, as in Li2MN (where M is a 3d-series metal such as Ni) and thereby reducing the energy barrier can also be studied; this kind of investigation is important for understanding potential
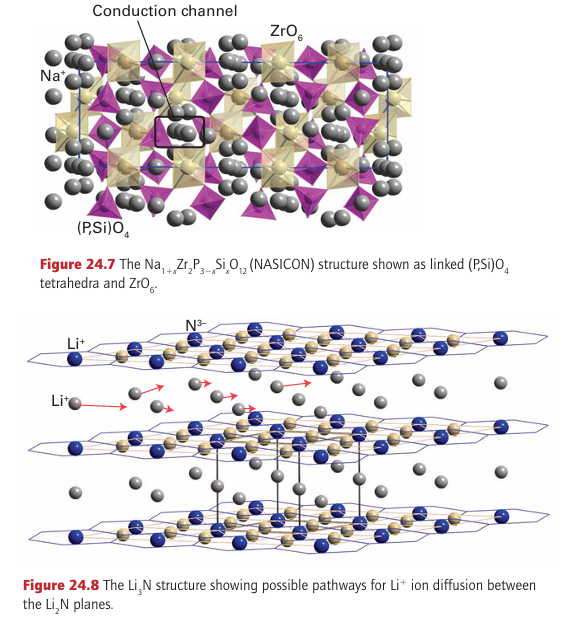
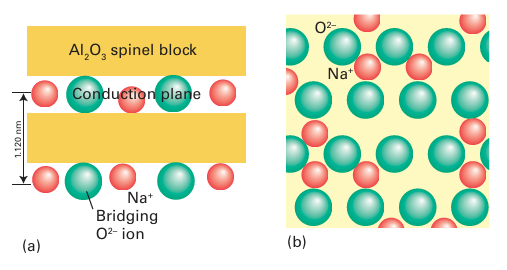
Figure 24.6 (a) Schematic side view of -alumina showing the Na2O conduction planes between Al2O3 slabs. The O atoms in these planes bridge the two slabs. (b) A view of the conduction plane. Note the abundance of mobile ions and vacancies in which they can move.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


