
Coordination compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص589-590
الجزء والصفحة:
ص589-590
 2025-10-07
2025-10-07
 259
259
Coordination compounds
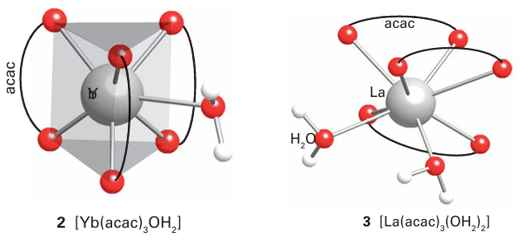
Key point: High coordination numbers with ligands adopting geometries that minimize interligand repulsions are the norm for the lanthanoids. The adoption by the relatively large, hard Lewis acid Ln3 ions of structures with high coordination numbers and with a variety of coordination environments in the solid state is repeated in solution. The variation in structure adopted is consistent with the view that the spatially buried f electrons have no significant stereochemical influence, and consequently ligands adopt positions that minimize interligand repulsions. In addition, polydentate ligands must satisfy their own stereochemical constraints, much as for the s-block ions and Al3 complexes. For example, many lanthanoid complexes have been formed with crown ether and -diketonate ligands. The coordination numbers for [Ln (OH2) n]3+ in aqueous solution are thought to be 9 for the early lanthanoids and 8 for the later, smaller members of the series, but these ions are highly labile and the measurements are subject to consider able uncertainty. Similarly, a striking variation is observed for the coordination numbers and structures of lanthanoid salts and complexes. For example, the small ytterbium cation, Yb3+, forms the seven-coordinate complex [Yb(acac)3(OH2)], and the larger La3+ is eight coordinate in [La(acac)3 (OH2)2]. The structures of these two complexes are approximately a capped trigonal prism (2) and a square antiprism (3), respectively. The partially fluorinated -diketonate ligand [C3F7 COCHCOC(CH3)3]−, nicknamed fod, produces complexes with Ln3 that are volatile and soluble in organic solvents. On account of their volatility, these complexes can be used as precursors for the synthesis of lanthanoid-containing materials by vapour deposition, such as the high temperature superconductors (Section 25.5).
Charged ligands generally have the highest affinity for the smallest Ln3 ion, and the re sulting increase in formation constants from large, lighter Ln3 (on the left of the series) to small, heavier Ln3 (on the right of the series) provides a convenient method for the chromatographic separation of these ions (Fig. 23.8). In the early days of lanthanoid chemistry, before ion-exchange chromatography was developed, tedious repetitive crystallizations were used to separate the elements. Lanthanoid complexes have found applications as shift reagents in NMR spectroscopy. The chemical shift of a proton (Section 8.5) is markedly shifted when it is in the vicinity of a paramagnetic centre and the -range considerably expanded. Hence addition of a small amount of a paramagnetic lanthanoid complex to a solution of a complex organic molecule results in marked changes to the spectrum as the two molecules are in close proximity in the solvent. This technique is of considerable use when NMR spectra are collected on low- and mid-field instruments as the resonances are spread out over a greater range of values, effectively increasing the resolution of the instrument by separating otherwise overlapping resonances. Europium and Yb complexes generally induce a downfield shift whereas Pr and Dy produce up field shifts. Commonly used shift reagents include Ln(fod)3 with Ln=Eu, Pr, and Yb. Chiral lanthanide shift reagents can be used to undertake an assay of enantiomeric composition as the NMR resonances of the two enantiomers are different and their separation improved in the presence of the shift reagent, Section 27.20.
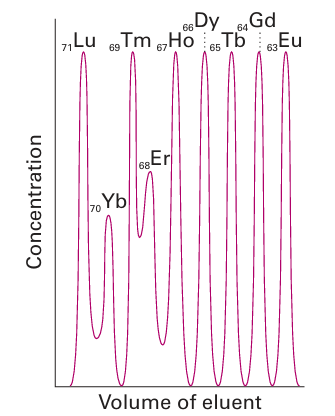
Figure 23.8 Elution of heavy lanthanoid ions from a cation exchange column using ammonium 2-hydroxyisobutyrate as the eluent. Note that the higher atomic number lanthanoids elute first because they have smaller radii and are more strongly complexed by the eluent.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


