
Ternary and complex oxides
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص588-589
الجزء والصفحة:
ص588-589
 2025-10-07
2025-10-07
 236
236
Ternary and complex oxides
Key point: Lanthanoid ions are often found in perovskites and garnets, where the ability to change the size of the ion allows the properties of the materials to be modified. The lanthanoids are a good source of stable, large, tripositive cations with a reasonable range of ionic radii. As a result, they can take one or more of the cation positions in ternary and more complex oxides. For example, perovskites of the type ABO3 can readily be pre pared with La on the A cation site; an example is LaFeO3. Indeed, some distorted structure types are named after lanthanoids; an example is the structural type GdFeO3 (Fig. 23.6), which has vertex-linked FeO6 octahedra around the Gd3 ion (as in the parent perovskite structure, Fig. 3.9) but the octahedra are tilted relative to each other. This tilting allows better coordination to the central Gd3 ion. The ability to change the size of the B3+ ion in a series of compounds LnBO3 allows the physical properties of the complex oxide to be modified in a controlled manner. For example, in the series of compounds LnNiO3 for Ln Pr to Eu, the insulating metallic transition temperature TIM (at which the properties change from that of a metal to that of an insulator, Section 3.19) increases with decreasing lanthanoid ionic radius:
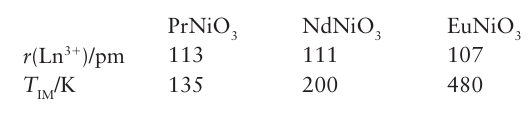
As the perovskite unit cell is a structural building block often found in more complex oxide structures, lanthanoids are frequently used in such materials. Famous examples are the original high-temperature superconducting cuprate (HTSC) La1.8 Ba0.2 CuO4 and the family of ‘123’ complex oxides, LnBa2 Cu3O7, which become superconducting below 93 K. The best known of these HTSCs is the d-block (yttrium) compound YBa2 Cu3O7, but they are also found for all the lanthanoids (Section 24.8). Another system where choice of lanthanoid is crucial in obtaining the required property is the complex manganites Ln1 xSrx MnO3 that exhibit resistance effects strongly dependent on the applied magnetic field and temperature; the optimized properties are found when Ln=Pr.
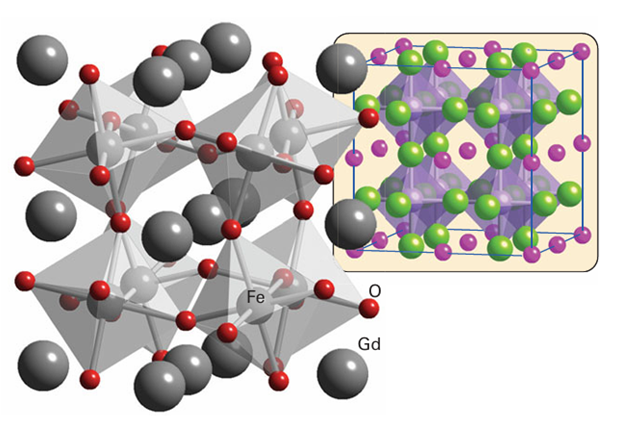
Figure 23.6 The GdFeO3 structure type, with the FeO6 octahedra outlined. The inset shows its relation to the ideal perovskite structure.
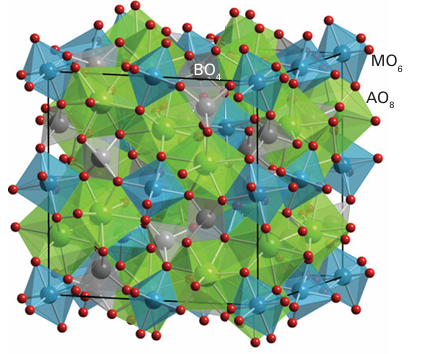
Figure 23.7 The garnet structure shown in the form of linked AO8 , BO4 , and MO6 polyhedra. The eight-coordinate A sites often occupied by yttrium can be occupied by other lanthanoids.The spinel structure (Fig. 3.44) has only small tetrahedral and octahedral holes in the close-packed O2 ion array and cannot accommodate bulky lanthanoid ions. However, the garnet structure adopted by materials of stoichiometry M3M2 (XO4)3, where M and M are normally di- and tripositive cations and X includes Si, Al, Ga, and Ge, has eight coordinate sites that can be occupied by lanthanoid ions, (Fig. 23.7). Yttrium aluminium garnet (YAG) is the host material for neodymium ions in the laser material Nd: YAG. Here we see a result of the similarities in the chemistry of yttrium from Group 3 and many lanthanoids. The contraction in the ionic radius of the lanthanoids with increasing atomic number means that many Ln3 ions have an ionic radius similar to that of Y3+ and therefore partial replacement of this ion by a lanthanide ion (for instance, Nd3+ in Nd: YAG) is easily achieved.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


