
Synthesis and reactivity of cyclopentadienyl compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص560-561
الجزء والصفحة:
ص560-561
 2025-10-04
2025-10-04
 330
330
Synthesis and reactivity of cyclopentadienyl compounds
Key points: Deprotonation of cyclopentadiene gives a convenient precursor to many metal cyclopentadienyl compounds; bound cyclopentadienyl rings behave as aromatic compounds and will undergo Friedel Crafts electrophilic reactions. Sodium cyclopentadienide, NaCp, is a common starting material for the preparation of cyclopentadienyl compounds. It can conveniently be prepared by the action of metallic sodium on cyclopentadiene in tetrahydrofuran solution:
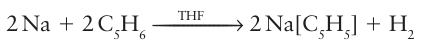
Sodium cyclopentadienide can then be used to react with d-metal halides to produce metallocenes. Cyclopentadiene itself is acidic enough that potassium hydroxide will deprotonate it in solution and, for example, ferrocene can be prepared with a minimum of fuss:
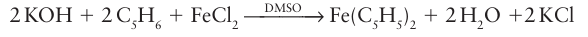
Because of their great stability, the 18-electron Group 8 compounds ferrocene, ruthenocene, and osmocene maintain their ligand metal bonds under rather harsh conditions, and it is possible to carry out a variety of transformations on the cyclopentadienyl ligands. For example, they undergo reactions similar to those of simple aromatic hydrocarbons, such as Friedel Crafts acylation:
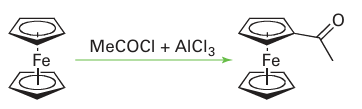
It also is possible to replace H on a C5H5 ring by Li:
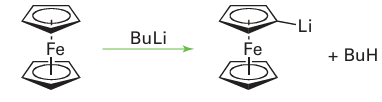
As might be imagined, the lithiated product is an excellent starting material for the synthesis of a wide variety of ring-substituted products and in this respect resembles simple organo lithium compounds (Section 11.17). Most Cp complexes of other metals undergo reactions similar to these two types where the five-membered ring behaves as an aromatic system.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


