
Alkanes, agostic hydrogens, and noble gases
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
552
الجزء والصفحة:
552
 2025-10-01
2025-10-01
 331
331
Alkanes, agostic hydrogens, and noble gases
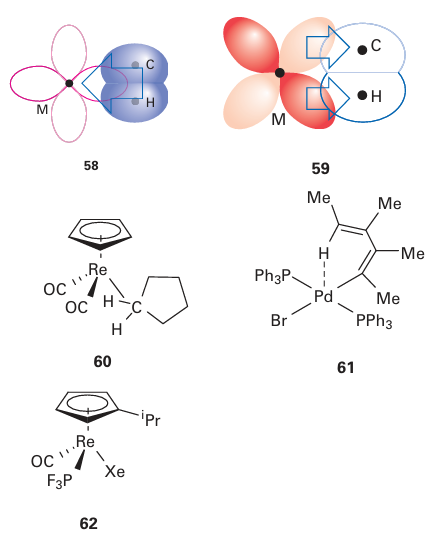
Key point: Alkanes can donate the electron density from C-H single bonds to a metal atom and, in the absence of other donors, even the electron density of a noble gas atom can enable it to behave as a ligand.
Highly reactive metal intermediates can be generated by photolysis and, in the absence of any other ligands, alkanes and noble gases have been observed to coordinate to the metal atom. Such species were first identified in the 1970s in solid methane and noble gas matrices, and were initially regarded as mere curiosities. However, both species have recently been fully characterized in solution and are now accepted as important intermediates in some reactions.
Alkanes are considered to donate electron density from a C-H σ bond to the metal atom (58), and accept π-electron density back from the metal atom into the corresponding σ* orbital (59), just like dihydrogen (Section 22.7). Although most alkane complexes are short lived, with the alkane being readily displaced, in 1998 the cyclopentane complex (60) was unambiguously identified in solution by NMR. Interactions between the C-H bond of an already coordinated ligand and the metal atom have also been observed. These species are referred to as having agnostic C-H inter actions, from the Greek for ‘to hold on to oneself’, and are thought to have additional stability due to the chelate effect (Section 7.14). Many examples of compounds with agnostic interactions are now known; an example is (61). Though weakly bonding, each C-H to metal atom interaction, whether it be agnostic or not, is considered formally to donate two electrons to the metal. Unlikely as it might seem, noble gas atoms can behave as ligands towards metal centres, and a number of complexes of Kr and Xe have been identified by IR spectroscopy, with the relatively long-lived Xe complex (62) characterized in solution by NMR in 2005. These complexes are stable only in the absence of better ligands (such as alkanes). The noble gas is formally considered to be neutral and donate two electrons.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


