
Carbenes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص551-552
الجزء والصفحة:
ص551-552
 2025-10-01
2025-10-01
 327
327
Carbenes
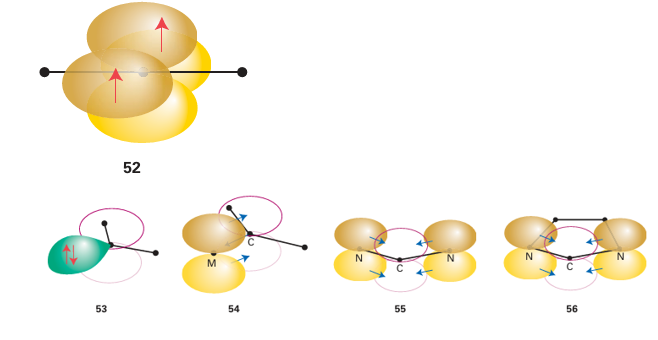
Key points: Fischer- and Schrock-type carbene complexes are considered to have a metal carbon double bond; N-heterocyclic carbenes are considered to have a metal carbon single bond, together with π backbonding. Carbene, CH2, has only six electrons around its C atom and is consequently highly reactive. Other substituted carbenes exist and are substantially less reactive and can behave as ligands towards metals. In principle, carbenes can exist in one of two electronic configurations: with a linear arrangement of the two groups bound to the carbon atom and the two remaining electrons unpaired in two p orbitals (52), or with the two groups bent, the two remaining electrons paired, and an empty p orbital (53). Carbenes with a linear arrangement of groups are re ferred to as ‘triplet carbenes’ (because the two unpaired electrons are unpaired and S=1) and are favoured when sterically very bulky groups are attached to the carbene carbon. Carbenes with the bent arrangement are known as ‘singlet carbenes’ (the two electrons are paired and S=0) and are the normal form for carbenes. The electron pair on the carbon atom of a singlet carbene is suitable to bond to a metal atom, resulting in a ligand-to-metal bond. The empty p orbital on the C atom can then accept electron density from the metal atom, thus stabilizing the electron-poor carbon atom (54). For historical reasons, carbenes bonded to metal atoms in this fashion are known as Fischer carbenes and are represented by a metal carbon double bond. Fischer carbenes are electron deficient at the C atom and consequently are easily attacked by nucleophiles. When the backbonding to the C atom is very strong, the carbene can become electron rich and is thus prone to attack by electrophiles. Carbenes of this type are known as Schrock carbenes, after their discoverer. The term alkylidene technically refers only to carbenes with alkyl substituents (CR2), but is sometimes used to mean both Fischer and Schrock carbenes.
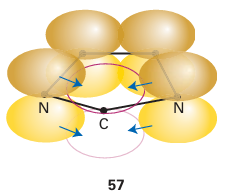
More recently, a large number of derivatives of what are known as N-heterocyclic carbenes (NHCs) have been used as ligands. In most NHCs, two nitrogen atoms are adjacent to the carbene carbon atom, and if the lone pair on the nitrogen is considered to be largely p-orbital based, then strong π-donor interactions from the two nitrogen atoms can help to stabilize the carbene (55). Tying the carbene C atom and the two N atoms into a ring helps to stabilize the carbene, and five-membered rings are common (56). Additional stability can be achieved by a double bond in the ring, which provides an additional two electrons that may be considered part of a six-electron aromatic resonance structure (57).
NHC ligands are considered to be two-electron σ donors and initial descriptions of the bonding suggested only minimal π backbonding from the metal atom. However, the current view is that there is actually significant π backbonding from the metal atom to the NHC.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


