
Cyclopentadiene and cycloheptatriene
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص550-551
الجزء والصفحة:
ص550-551
 2025-10-01
2025-10-01
 357
357
Cyclopentadiene and cycloheptatriene
Key points: The common η5-bonding mode of a cyclopentadienyl ligand can be understood on the basis of both σ and π donation from the organic fragment to the metal in conjunction with δ back bonding; cycloheptatriene commonly forms either η6-complexes or η7-complexes of the aromatic tropilium cation (C7H7) +.
Cyclopentadiene, C5H6, is a mildly acidic hydrocarbon that can be deprotonated to form the cyclopentadienyl anion, C5H5. The stability of the cyclopentadienyl anion can be understood when it is realized that the six electrons in its π system make it aromatic. The delocalization of these six electrons results in a ring structure with five equal bond lengths. As a ligand, the cyclopentadienyl group, commonly denoted Cp, has played a major role in the development of organometallic chemistry and continues to be the archetype of cyclic polyene ligands. We have already alluded to the role ferrocene (4) had in the development of organometallic chemistry. A huge number of metal cyclopentadienyl and substituted cyclopentadienyl compounds are known. Some compounds have C5H5 as a monohapto ligand (12), in which case it is treated like an η1-alkyl group; others contain C5H5 as a trihapto ligand (13), in which case it is treated like an η3-allyl group. Usually, though, C5H5 is present as a pentahapto ligand, bound through all five carbons of its ring. We treat the 5-C5H5 group as a six-electron donor. Formally, the electron donation to the metal now comes from the filled 1π (bonding) and 2π (π bonding) MOs (Fig. 22.10) with Ϭ back bonding to the dxy and dx2y2 orbitals on the metal atom. As we shall see in Section 22.19, coordinated Cp ligands behave as though they maintain their six-electron aromatic structure.
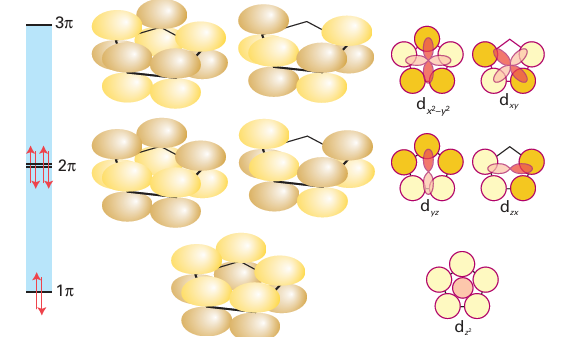
Figure 22.10 The molecular orbitals for the π systems of the cyclopentadienyl group; also shown are metal d orbitals of appropriate symmetry to form bonding interactions.
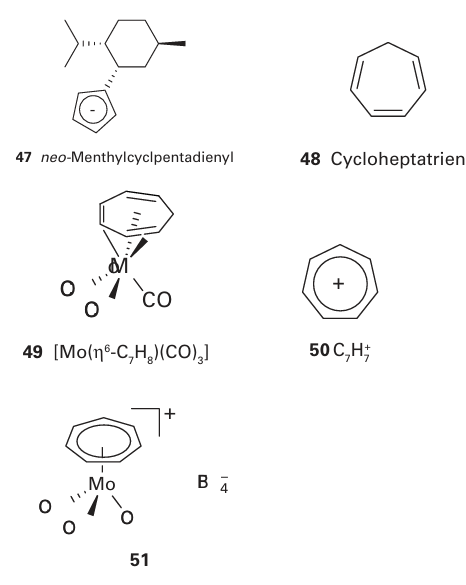
The electronic and steric properties of cyclopentadiene can easily be tuned: electron withdrawing and donating groups can be attached to the five-membered ring, and steric bulk can be enhanced by additional substitution. The pentamethylcyclopentadienyl ligand (Cp*) is commonly used to provide greater electron density on, and greater steric protection of, a metal atom. Chiral groups are often added to Cp groups so that complexes can be used in stereoselective reactions: the neo-menthyl group (47) is commonly used. The synthesis, properties, and reactivities of compounds containing the cyclopenta- dienyl ligand are discussed in more detail in Section 22.19. Cycloheptatriene, C7H8+ (48), can form η6-complexes such as (49), which may be treated as having three η2-alkene molecules bound to the metal atom. Hydride abstraction from these complexes results in formation of η7-complexes of the six-electron aromatic cation C7H7+ (50), for example (51). In η7-cycloheptatrienyl complexes, all carbon-carbon bond lengths are equal; bonding to the metal atom and back bonding from the metal are similar to those found in arene and cyclopentadienyl complexes.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


