
Nomenclature
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص539-540
الجزء والصفحة:
ص539-540
 2025-09-30
2025-09-30
 344
344
Nomenclature
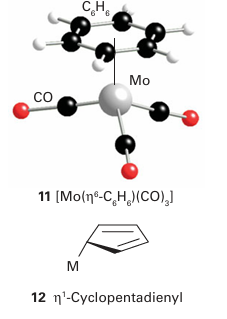
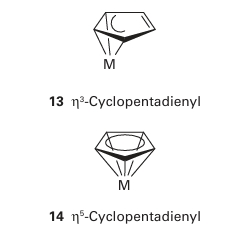
Key point: The naming of organometallic compounds is similar to the naming of coordination com pounds, but certain ligands have multiple bonding modes, which is reported as the hapticity. According to the recommended convention, we use the same system of nomenclature for organometallic compounds as set out for coordination complexes in Section 7.2. Thus, ligands are listed in alphabetical order followed by the name of the metal, all of which is written as one word. The name of the metal should be followed by its oxidation number in parentheses. The nomenclature used in research journals, however, does not always obey these rules, and it is common to find the name of the metal buried in the mid dle of the name of the compound and the oxidation number omitted. For example, (11) is sometimes referred to as benzene molybdenum-tricarbonyl, rather than the preferred name benzene(tricarbonyl)molybdenum (0). The IUPAC recommendation for the formula of an organometallic compound is to write it in the same form as for a coordination complex: the symbol for the metal is written first, followed by the ligands, listed in alphabetical order based on their chemical symbol. We shall follow these conventions unless a different order of ligands helps to clarify a particular point. Often a ligand with carbon donor atoms can exhibit multiple bonding modes—for instance, the cyclopentadienyl group can commonly bond to a d-metal atom in three different ways—thus, we need some additional nomenclature. Without going into the intimate details of the bonding of the various ligands (we do that later in this chapter), the extra information we need to describe a bonding mode is the number of points of attachment. This procedure gives rise to the notion of hapticity, the number of ligand atoms that are considered formally to be bonded to the metal atom. The hapticity is denoted ηn, where n is the number of atoms (and η is eta). For example, a CH3 group attached by a single MC bond is monohapto, η1, and if the two C atoms of an ethene ligand are both within bonding distance of the metal, the ligand is dihapto, η2. Thus, three cyclopentadienyl complexes might be described as having η1 (12), η3 (13), or η5 (14) cyclopentadienyl groups.
Some ligands (including the simplest of them all, the hydride ligand, H) can bond to more than one metal atom in the same complex, and are then referred to as bridging ligands. We do not require any new concepts to understand bridging ligands other than those introduced in Section 2.11. Recall from Section 7.2 that the Greek letter u (mu) is used to indicate how many atoms the ligand bridges. Thus a 2-CO is a carbonyl group that bridges two metal atoms and a 3-CO bridges three.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


