
The trans effect
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص514-515
الجزء والصفحة:
ص514-515
 2025-09-29
2025-09-29
 299
299
The trans effect
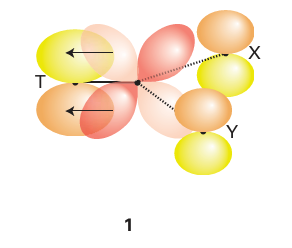
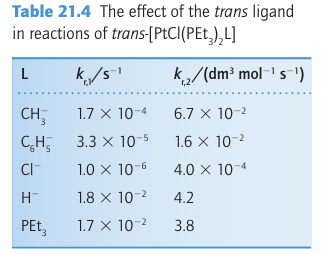
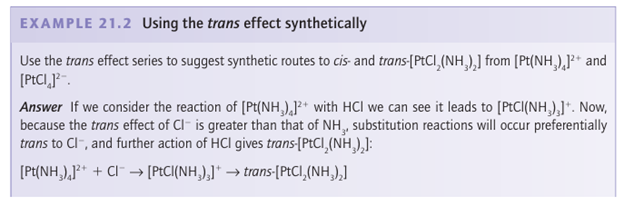
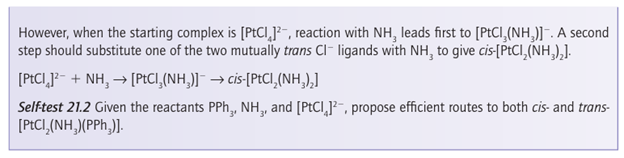
Key point: A strong -donor ligand or π-acceptor ligand greatly accelerates substitution of a ligand that lies in the trans position. The spectator ligands T that are trans to the leaving group in square-planar complexes influence the rate of substitution. This phenomenon is called the transeffect. It is generally accepted that the trans effect arises from two separate influences: one arising in the ground state and the other in the transition state itself. Thetransinfluence is the extent to which the ligand T weakens the bond trans to itself in the ground state of the complex. The trans influence correlates with the -donor ability of the ligand T because, broadly speaking, ligands trans to each other use the same orbitals on the metal for bonding. Thus if one ligand is a strong donor, then the ligand trans to it cannot donate electrons to the metal so well, and thus has a weaker interaction with the metal. The trans influence is assessed quantitatively by measuring bond lengths, stretching frequencies, and metal-to-ligand NMR coupling constants (Section 8.5). The transition state effect correlates with the π-acceptor ability of the ligand. Its origin is thought to be the increase in electron density on the metal atom arising due to the incoming ligand: any ligand that can accept this increased electron density will stabilize the transition state (1). The trans effect is the combination of both effects; it should be noted that the same factors contribute to a large ligand-field splitting. Trans effects are listed in Table 21.4 and follow the order:
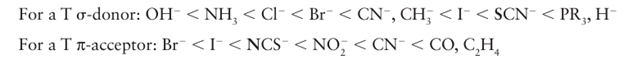
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


