
Hydrides of sulfur, selenium, and tellurium
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
407
الجزء والصفحة:
407
 2025-09-25
2025-09-25
 321
321
Hydrides of sulfur, selenium, and tellurium
Key points: The extent of hydrogen bonding is much less for these hydrides than for water; all the hydrides are gases. Hydrogen sulfide, H2S, is toxic, its toxicity made more hazardous by the fact that it tends to anaesthetize the olfactory nerves, making intensity of smell a dangerously inaccurate guide to con centration. Hydrogen sulfide is produced by volcanoes and by some micro-organisms (Box 16.3). It is an impurity in natural gas and must be removed before the gas is used. Pure H2S can be prepared by direct combination of the elements above 600C:
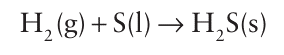
Hydrogen sulfide is easily generated in the laboratory by trickling dilute hydrochloric or phosphoric acid on to FeS:
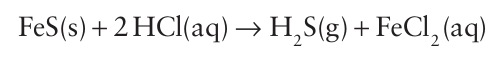
It can also be prepared by hydrolysis of aluminium sulfide, which is easily generated by ignition of a mixture of the elements:
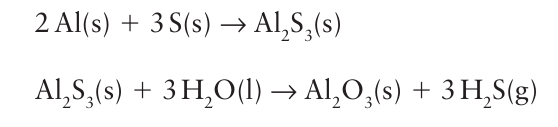
It is readily soluble in water, and is a weak acid:
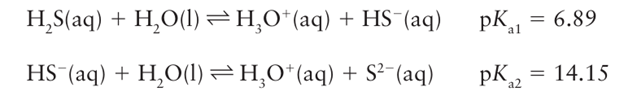
Acidic solutions of H2S are mild reducing agents and deposit elemental S on standing. In a similar way, H2 Se can be made by direct combination of the elements, by the reaction of FeSe with hydrochloric acid, or by hydrolysis of Al2Se3:
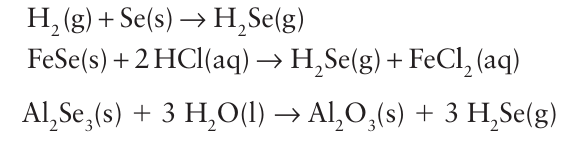
On the other hand, H2 Te is made by hydrolysis of Al2Te3 or by the action of hydrochloric acid on Mg, Zn, or Al tellurides.
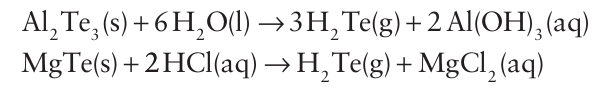
The solubilities of H2Se and H2 Te in water are similar to that of H2S. The acidity constants of the hydrides (which are protic) increase from H2S to H2 Te (Table 16.2). Like their sulfur analogue, aqueous solutions of H2 Se and H2 Te are readily oxidized and deposit elemental selenium and tellurium on standing.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


