
Intermediate oxidation states in the 4d and 5d series
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
456
الجزء والصفحة:
456
 2025-09-23
2025-09-23
 231
231
Intermediate oxidation states in the 4d and 5d series
Key points: Complexes of M(II) with -donor ligands are common for the 3d-series metals but complexes of M(II) of the 4d- and 5d-series metals are less common; they generally contain -acceptor ligands.
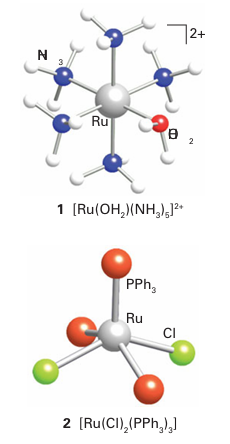
In contrast to the 3d-series, the 4d- and 5d-series metals only rarely form simple M2 (aq) ions. A few examples have been characterized, including [Ru (OH2)6]2+, [Pd (OH2)4]2+, and [Pt (OH2)4]2+. However, the 4d- and 5d-series metals do form many M(II) complexes with ligands other than H2 O; they include the very stable d6 octahedral complexes, such as (1), and the much rarer square-pyramidal d6 complexes, such as (2), which form with bulky ligands. Palladium (II) and platinum (II) form many square-planar d8 complexes, such as [PtCl4]2. The Ru (II) complex in (1) is obtained by reducing RuCl3 .3H2O with zinc in the presence of ammonia; it is a useful starting material for the synthesis of a range of ruthenium (II) pentaamine complexes that have -acceptor ligands, such as CO, as the sixth ligand:
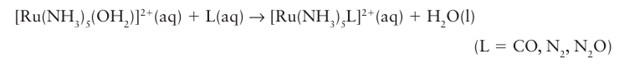
These Ru and the related Os pentaamine species are strong donors, and consequently the complexes they form with the acceptors CO and N2 are stable.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


