
Selenium, tellurium, and polonium
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص405-406
الجزء والصفحة:
ص405-406
 2025-09-10
2025-09-10
 410
410
Selenium, tellurium, and polonium
Key points: Selenium and tellurium crystallize in helical chains; polonium crystallizes in a primitive cubic form. Selenium can be extracted from the waste sludge from sulfuric acid plants. Selenium and tellurium can be extracted from copper sulfide ores, where they occur as the copper selenide or telluride. The extraction method depends on the other compounds or elements present. The first step usually involves oxidation in the presence of sodium carbonate:
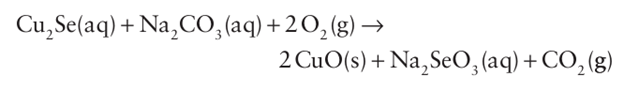
The solution containing Na2 SeO3 and Na2 TeO3 is acidified with sulfuric acid. The Te precipitates out as the dioxide, leaving selenous acid, H2SeO3, in solution. Selenium is recovered by treatment with SO2:
=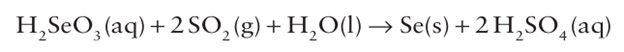 Tellurium is liberated by dissolving the TeO2 in aqueous sodium hydroxide followed by electrolytic reduction:
Tellurium is liberated by dissolving the TeO2 in aqueous sodium hydroxide followed by electrolytic reduction:
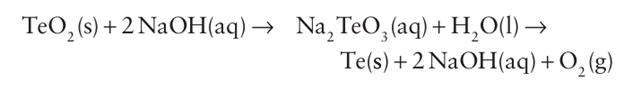
As with S, three polymorphs of Se exist that contain Se8 rings and differ only in the packing of the rings to give α,ß, and y forms of red selenium. The most stable form at room tempera ture is metallic grey selenium, a crystalline material composed of helical chains. The common commercial form of the element is amorphous black selenium; it has a very complex structure comprising rings containing up to 1000 Se atoms. Another amorphous form of Se, obtained by deposition of the vapour, is used as the photoreceptor in the xerographic photocopying process. Selenium is an essential element for humans, but, as with many essential elements, there is only a narrow range of concentration between the minimum daily requirement and toxicity. An early indication of Se poisoning is a garlicky smell on the breath, which is due to methylated selenium. Selenium exhibits both photovoltaic character, where light is converted directly into electricity, and photoconductive char acter. The photoconductivity of grey selenium arises from the ability of incident light to excite electrons across its reasonably small band gap (2.6 eV in the crystalline material, 1.8 eV in the amorphous material). These properties make Se useful in the production of photocells and exposure meters for photographic use, as well as solar cells. Selenium is also a p-type semiconductor (Section 3.20) and is used in electronic and solid-state applications. It is also used in photocopier toner and in the glass industry to make red glasses and enamels.
Tellurium crystallizes in a chain structure like that of grey selenium. Polonium crystallizes in a primitive cubic structure and a closely related higher temperature form above 36C. We remarked in Section 3.5 that the primitive cubic structure rep resents inefficient packing of atoms, and Po is the only element that adopts this structure under normal conditions. Tellurium and Po are both highly toxic; the toxicity of Po is enhanced by its intense radioactivity. Mass for mass, it is about 2.5 1011 times as toxic as hydrocyanic acid. All 29 isotopes of polonium are radioactive. It has been found in tobacco as a contaminant and in uranium ores. It can be produced in small amounts (gram quantities) through irradiation of 209Bi (atomic number 83) with neutrons, which gives 210Po (atomic number 84):
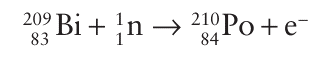
Metallic Po can then be separated from the remaining Bi by fractional distillation or electrodeposited on to a metal surface. Selenium, Te, and Po combine directly with most elements, although less readily than O or S. The occurrence of multiple bonds is lower than with O or S, as is the tendency towards catenation (compared with S) and the number of allotropes. The unexpected difficulty of oxidizing Se to Se (VI) (Fig. 16.2) is due to the lanthanide-like contraction (Section 9.2a) in radius following the 3d elements.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


