
Phosphides
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص382-383
الجزء والصفحة:
ص382-383
 2025-09-07
2025-09-07
 363
363
Phosphides
Key point: Phosphides may be metal-rich or phosphorus-rich. The compounds of phosphorus with hydrogen, oxygen, and the halogens are discussed separately. The phosphides of other elements can be prepared by heating the appropriate element with red phosphorus in an inert atmosphere:
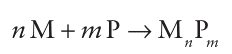
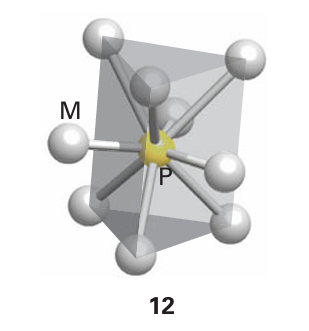
There are many varieties of phosphides, with formulas ranging from M4 P to MP15. They include metal-rich phosphides, in which M:P > 1, monophosphides, in which M:P = 1, and phosphorus-rich phosphides, in which M:P < 1. Metal-rich phosphides are usual ly very inert, hard, brittle refractory materials and resemble the parent metal in having high electrical and thermal conductivities. The structures have a trigonal prismatic arrangement of six, seven, eight, or nine metal ions around a P atom (12). Monophosphides adopt a variety of structures depending on the relative size of the other atom. For example, AlP adopts the zinc-blende structure, SnP adopts the rock-salt structure, and VP adopts the nickel-arsenide structure (Section 3.9). Phosphorus-rich phosphides have lower melting points and are less stable than metal-rich phosphides and monophosphides. They are semiconductors rather than conductors.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


