
Enthalpies of atomization
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص260-261
الجزء والصفحة:
ص260-261
 2025-09-03
2025-09-03
 490
490
Enthalpies of atomization
Key point: The enthalpy of atomization increases with increasing number of valence electrons. The enthalpy of atomization of an element, ∆aHO, is a measure of the energy required to form gaseous atoms. For solids, the enthalpy of atomization is the enthalpy change
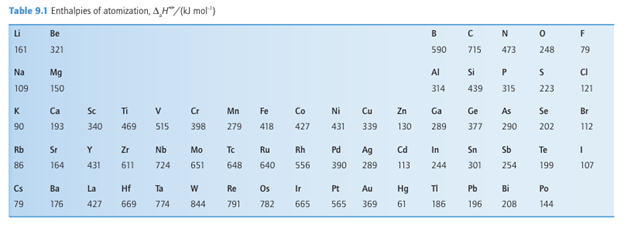
associated with the atomization of the solid; for molecular species, it is the enthalpy of dissociation of the molecules. As can be seen in Table 9.1, enthalpies of atomization first increase and then decrease across Periods 2 and 3, reaching a maximum at C in Period 2 and Si in Period 3. The values decrease between C and N, and Si and P: even though N and P each have five valence electrons, two of these electrons form a lone pair and only three are involved in bonding. A similar effect is seen between N and O, where O has six valence electrons of which four form lone pairs and only two are involved in bonding. These trends are shown in Fig. 9.7. The enthalpies of atomization of the d-block elements are higher than those of the s- and p-block elements, in line with their greater number of valence electrons and consequently stronger bonding. The values reach a maximum at Groups 5 and 6 (Fig. 9.8), where there is a maximum number of unpaired electrons available to form bonds. The middle of each row shows an irregularity due to spin correlation (Section 1.7a), which favours a half filled d shell for the free atom. This effect is particularly evident for the 3d series, in which Cr (3d54s1) and Mn (3d54s2) have significantly lower atomization energies than expected from a simple consideration of their number of valence electrons. The enthalpy of atomization decreases down a group in the s and p blocks but increases down a group in the d block. Thus s and p orbitals become less effective at forming bonds as the period number increases, whereas d orbitals become more effective. These trends are attributed to the expansion of p orbitals on descending a group from optimal for overlap to too diffuse for extensive overlap and, in contrast, d orbitals expanding in size from too contracted to optimal for overlap. The same trends can be seen in the melting points (Table 9.2) of the elements, where a greater number of valence electrons leads to greater binding energy and a higher melting temperature.
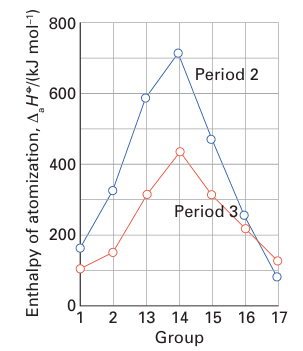
Figure 9.7 Variation of the enthalpy of atomization in the s- and p-block elements.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


