
Electrochemical techniques
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص249-250
الجزء والصفحة:
ص249-250
 2025-09-03
2025-09-03
 390
390
Electrochemical techniques
Key point: Cyclic voltammetry measures the electrical currents due to reduction and oxidation of electroactive species in solution. Incyclic voltammetry the current flowing between two electrodes immersed in a solution is measured as the potential difference is changed cyclically. It provides direct information on reduction potentials and the stabilities of different products of oxidation or reduction. The technique gives rapid qualitative insight into the redox properties of an electroactive com pound and reliable quantitative information on its thermodynamic and kinetic properties. The ‘working electrode’ at which the electrochemical reaction of interest occurs is usually constructed from platinum, silver, gold, or graphite. The reference electrode is normally a silver/silver-chloride electrode and the counter electrode is normally platinum. To understand what is involved, consider the redox couple [Fe (CN)6]3–/[Fe(CN)6]4–, where, initially, only the reduced form (the Fe (II) complex) is present (Fig. 8.46). The con centration of electroactive species is usually quite low (less than 0.001 m) and the solution contains a relatively high concentration of inert ‘supporting’ electrolyte (at concentrations greater than about 0.1 m) to provide conductivity. The potential difference is applied between the working electrode and the reference electrode and is scanned back and forth between two limits, tracing out a triangular waveform. No current flows while the potential is low. As it approaches the reduction potential of the Fe (III)/Fe (II) couple, the Fe (II) is oxidized at the working electrode and a current starts to flow. This current rises to a peak then decrease steadily because Fe (II) becomes depleted close to the electrode (the solution is unstirred) and must be supplemented by species diffusing from increasingly distant regions of the solution. Once the upper potential limit is reached, the potential sweep is reversed. Initially, Fe (II) diffusing to the electrode continues to be oxidized but eventually the potential difference becomes sufficiently negative to re duce the Fe (III) that had been formed; the current reaches a peak then decreases gradually to zero as the lower potential limit is reached . The average of the two peak potentials is a good approximation to the reduction potential E under the prevailing conditions (E is not in general the standard potential because nonstandard conditions are usually adopted). In the ideal case, the oxidation and reduction peaks are similar in magnitude and separated by a small potential increment, which is usually (59 mV)/ e at 25ºC, where e is the number of electrons transferred during the reaction at the electrode. This case is an example of a reversible redox reaction, with electron transfer at the electrode sufficiently fast that equilibrium is always maintained throughout the potential sweep. In such a case the current is usually limited by diffusion of electroactive species to the electrode. Slow kinetic processes at the electrode result in a large separation of reduction and oxidation peaks that increases with increasing scan rate. This separation arises because an overpotential (Section 5.18, effectively a driving force) is required to overcome barriers to electron transfer in each direction. Moreover, the peak due to a reduction or an oxidation in the initial part of the cycle process is often not matched by a corresponding peak in the reverse direction. This absence occurs because the species that is initially generated undergoes a further chemical reaction during the cycle and produces either a species with a different reduction potential or one that is not electroactive within the range of potential scanned. Inorganic chemists often refer to this behaviour as ‘irreversible’. An electrochemical reaction followed by a chemical reaction is known as an EC process. By analogy, a CE process is a reaction in which the species able to undergo the electro chemical (E) reaction must first be generated by a chemical reaction. Thus, for a molecule that is suspected of decomposing upon oxidation, it may be possible to observe the initial unstable species formed by the E process provided the scan rate is sufficiently fast to re-reduce it before it undergoes further reaction. Consequently, by varying the scan rate, the kinetics of the chemical reaction can be determined.
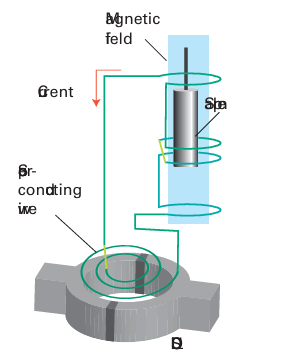
Figure 8.45 The magnetic susceptibility of a sample is measured by using a SQUID: the sample, which is exposed to a magnetic field, is moved through the loops in small increments and the potential difference across the SQUID is monitored.
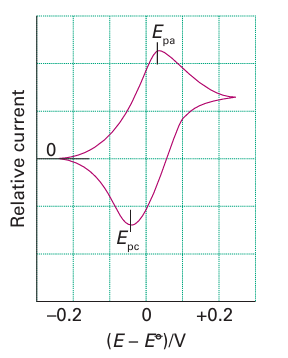
Figure 8.46 The cyclic voltammogram for an electroactive species present in solution as the reduced form and displaying a reversible one-electron reaction at an electrode. The peak potentials Epa and Epc for oxidation and reduction, respectively, are separated by 0.06 V. The reduction potential is the mean of Epa and Epc.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


