
Extended silicon oxygen compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص353-354
الجزء والصفحة:
ص353-354
 2025-08-31
2025-08-31
 282
282
Extended silicon oxygen compounds
Key point: As well as forming simple binary compounds with oxygen, silicon forms a wide range of extended network solids that find a range of applications in industry.
Aluminosilicates are formed when Al atoms replace some of the Si atoms in a silicate and occur naturally as clays, minerals, and rocks. Zeolite aluminosilicates are widely used as molecular sieves, microporous catalysts, and catalyst support materials. Because Al occurs as Al (III), its presence in place of Si (IV) in an aluminosilicate renders the overall charge
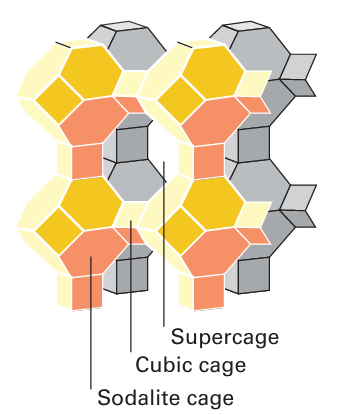
Figure 14.3 Framework representation of a type-A zeolite. Note the sodalite cages (truncated octahedral), the small cubic cages, and the central supercage.
more negative by one unit. An additional cation, such as H, Na, or 1 2 Ca2, is therefore required for each Al atom that replaces an Si atom. These additional cations have a pro found effect on the properties of the materials. Many important minerals are varieties of layered aluminosilicates that also contain met als such as lithium, magnesium, and iron: they include clays, talc, and various micas. An example of a simple layered aluminosilicate is the mineral kaolinite, Al2(OH)4Si2O5 , which is used commercially as China clay and in some medical applications. It has long been used in diarrhea remedies and a more recent application uses kaolinite nanoparticle-impregnated bandages to stop bleeding, as the mineral triggers blood clotting. In the mineral talc, Mg3 (OH)2 Si4O10, Mg2 and OH ions are sandwiched between layers of Si4O104- anions. The arrangement is electrically neutral, and as a result talc readily cleaves between the layers and accounts for talc’s familiar slippery feel. Muscovite mica, KAl2(OH)2Si3 AlO10, has charged layers because one Al (III) atom substitutes for one Si (IV) atom and the resulting negative charge is compensated by a K ion that lies between the repeating layers. Because of this electrostatic cohesion, muscovite is not soft like talc but it is readily cleaved into sheets. There are many minerals based on a three-dimensional aluminosilicate framework. The feldspars, for instance, are the most important class of rock-forming minerals.
The molecular sieves are crystalline microporous aluminosilicates having open structures with apertures of molecular dimensions. The name ‘molecular sieve’ is prompted by the observation that these materials absorb only molecules that are smaller than the aperture dimensions and so can be used to separate molecules of different sizes. A subclass of molecular sieves, the zeolites,1 have an aluminosilicate framework with cations (typi cally from Groups 1 or 2) trapped inside tunnels or cages (Fig. 14.3). In addition to their function as molecular sieves, zeolites are used as ion-exchange resins as they can exchange their ions for those in a surrounding solution. Zeolites are also used for shape-selective heterogeneous catalysis.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


