
Hydrogen fluoride
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
130
الجزء والصفحة:
130
 2025-08-27
2025-08-27
 466
466
Hydrogen fluoride
Key point: Hydrogen fluoride is a reactive toxic solvent that is highly acidic. Liquid hydrogen fluoride (bp 19.5ºC) is an acidic solvent with a relative permittivity (εr=84 at 0°C) comparable to that of water (εr=78 at 25°C). It is a good solvent for ionic substances. However, as it is both highly reactive and toxic, it presents handling problems, including its ability to etch glass. In practice, liquid hydrogen fluoride is usually contained in polytetrafluoroethylene and polychlorotrifluoroethylene vessels. Hydrogen fluoride is particularly hazardous because it penetrates tissue rapidly and interferes with nerve function. Consequently, burns may go undetected and treatment may be delayed. It can also etch bone and reacts with calcium in the blood. Liquid hydrogen fluoride is a highly acidic solvent as it has a high autoprotolysis constant and produces solvated protons very readily (Section 4.1(b)):
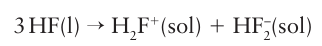
Although the conjugate base of HF is formally F–, the ability of HF to form a strong hydrogen bond to F– means that the conjugate base is better regarded as the bifluoride ion, HF2–. Only very strong acids are able to donate protons and function as acids in HF, for example fluorosulfonic acid:
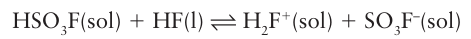
Organic compounds such as acids, alcohols, ethers, and ketones can accept a proton and act as bases in HF(l). Other bases increase the concentration of HF2 to produce basic solutions:
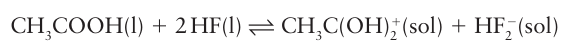
In this reaction acetic acid, an acid in water, is acting as a base. Many fluorides are soluble in liquid HF as a result of the formation of the HF2– ion; for example,
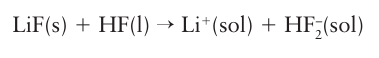
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


