
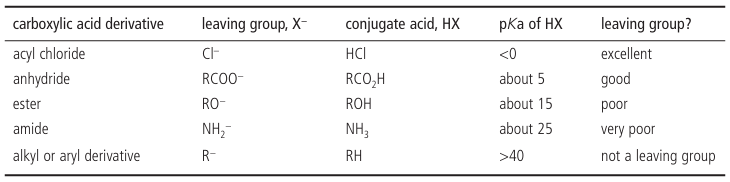
pKa is a useful guide to leaving group ability
 المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
 المصدر:
ORGANIC CHEMISTRY
المصدر:
ORGANIC CHEMISTRY
 الجزء والصفحة:
ص202
الجزء والصفحة:
ص202
 2025-05-10
2025-05-10
 775
775
It’s useful to be able to compare leaving group ability quantitatively. This is impossible to do exactly, but a good guide is the pKa of the conjugate acid (Chapter 8). If X− is the leaving group, the lower the pKa of HX, the better X− is as a leaving group. If we go back to the example of ester formation from acyl chloride plus alcohol, there’s a choice of Me−, EtO−, and Cl−. HCl is a stronger acid than EtOH, which is a much stronger acid than methane. So Cl− is the best leaving group and EtO− the next best. These observations apply only to reactions at the car bonyl group.
●Leaving group ability, The lower the pKa of HX, the better the leaving group of X− in carbonyl substitution reactions.
The most important substituents in carbonyl reactions are alkyl or aryl groups (R), amino groups in amides (NH2), alkoxy groups in esters (RO−), carboxylate groups (RCO2 −) in anhydrides, and chloride (Cl−) in acyl chlorides. The order of leaving group ability is then:
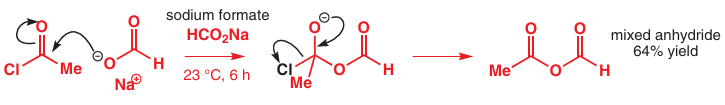
We can use pKa to predict what happens if we react an acyl chloride with a carboxylate salt. We expect the carboxylate salt (here, sodium formate or sodium methanoate, HCO2Na) to act as the nucleophile to form a tetrahedral intermediate, which could collapse in any one of three ways. We can straightaway rule out loss of Me− and we might guess that Cl− is a better leaving group than HCO2 − as HCl is a much stronger acid than a carboxylic acid, and we’d be right. Sodium formate reacts with acetyl chloride to give a mixed anhydride.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


