
Making primary alcohols from organometallics and formaldehyde
 المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
 المصدر:
ORGANIC CHEMISTRY
المصدر:
ORGANIC CHEMISTRY
 الجزء والصفحة:
ص191-192
الجزء والصفحة:
ص191-192
 2025-05-08
2025-05-08
 638
638
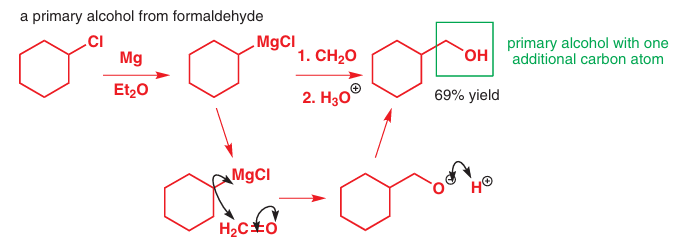
You met formaldehyde, the simplest aldehyde, in Chapter 6, where we discussed the difficulties of using it in anhydrous reactions: it is either hydrated or a polymer paraformaldehyde, (CH2O)n, and in order to get pure, dry formaldehyde it is necessary to heat (‘crack’) the polymer to decompose it. But formaldehyde is a remarkably useful reagent for making primary alcohols, in other words alcohols that have just one carbon substituent on the hydroxy- bearing C atom. Just as carbon dioxide adds one carbon and makes an acid, formaldehyde adds one carbon and makes an alcohol.
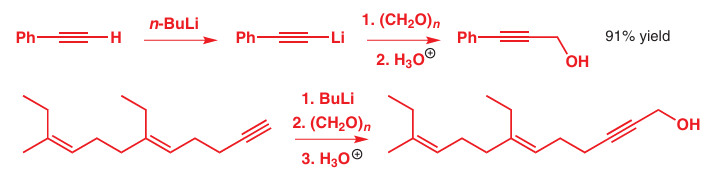
In the next two examples, formaldehyde makes a primary alcohol from two deprotonated alkynes. The second reaction here (for which we have shown organolithium formation, reac tion, and quench simply as a series of three consecutive reagents) forms one of the last steps of the synthesis of Cecropia juvenile hormone, whose structure you met right at the beginning of the chapter.
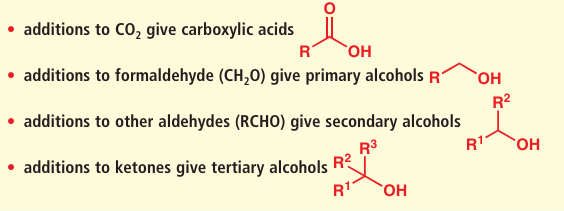
●Something to bear in mind with all organometallic additions to carbonyl compounds is that the addition takes the oxidation level down one (oxidation levels were described in Chapter 2, p. 33). In other words, if you start with an aldehyde, you end up with an alcohol. More specifically,
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


