
Making organometallics, How to make Grignard reagents
 المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
المؤلف:
Jonathan Clayden , Nick Greeves , Stuart Warren
 المصدر:
ORGANIC CHEMISTRY
المصدر:
ORGANIC CHEMISTRY
 الجزء والصفحة:
ص184-185
الجزء والصفحة:
ص184-185
 2025-05-07
2025-05-07
 657
657
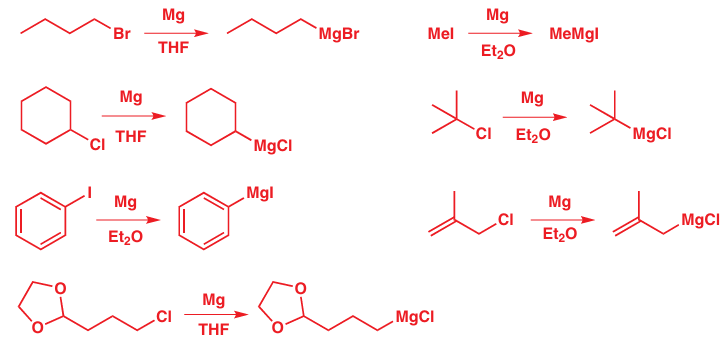
Grignard reagents are made by reacting magnesium turnings with alkyl halides in ether solvents to form solutions of alkyl magnesium halide. Iodides, bromides, and chlorides can be used, as can both aryl and alkyl halides. Our examples include methyl, primary, secondary, and tertiary alkyl halides, aryl and allyl halides. They cannot contain any functional groups that would react with the Grignard reagent once it is formed. The final example has an acetal functional group as an example of one that does not react with the Grignard reagent. (See Chapter 23 for further discussion.)
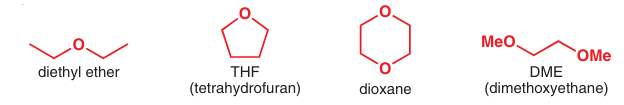
The solvents in these examples are all ethers, either diethyl ether Et2O or THF. Other solvents that are sometimes used include the diethers dioxane and dimethoxymethane (DME).
common ether solvents
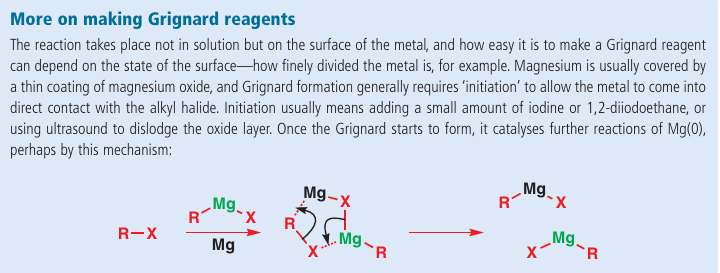
The reaction scheme is easy enough to draw, but what is the mechanism? Overall, it involves an insertion of magnesium into the carbon–halogen bond. There is also a change in oxidation state of the magnesium, from Mg (0) to Mg (II). The reaction is therefore known as an oxidative insertion or oxidative addition, and is a general process for many metals such as Mg, Li (which we meet shortly), Cu, and Zn. Mg (II) is much more stable than Mg (0) and this drives the reaction. The mechanism of the reaction is not completely understood, and probably involves radical intermediates. But what is sure is that by the end of the reaction the magnesium has surrendered its lone pair of electrons and gained two σ bonds. The true product is a complex between the Grignard reagent and, probably, two molecules of the ether solvent, as Mg (II) prefers a tetrahedral structure.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


