
Orbital energies in a hydrogen-like species
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 27-6-2019
27-6-2019
 1341
1341
Orbital energies in a hydrogen-like species
Besides providing information about the wave functions, solutions of the Schrodinger equation give orbital energies, E (energy levels), and equation 1.16 shows the dependence of E on the principal quantum number for hydrogen-like species.

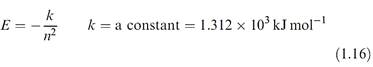
For each value of n there is only one energy solution and for hydrogen-like species, all atomic orbitals with the same principal quantum number (e.g. 3s, 3p and 3d ) are degenerate.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


