
Physical Properties of Sulfur
 المؤلف:
........
المؤلف:
........
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.......
الجزء والصفحة:
.......
 11-11-2018
11-11-2018
 2412
2412
Physical Properties of Sulfur
Sulfur has an atomic weight of 32.066 grams per mole and is part of group 16, the oxygen family. It is a nonmetal and has a specific heat of 0.706 J g-1 oC-1. The electron affinity if 200 kJ mol-1 and the electronegativity is 2.58 (unitless). Sulfur is typically found as a light-yellow, opaque, and brittle solid in large amounts of small orthorhombic crystals. Not only does sulfur have twice the density of water, it is also insoluble in water. On the other hand, sulfur is highly soluble in carbon disulfide and slightly soluble in many common solvents. Sulfur can also vary in color and blackens upon boiling due to carbonaceous impurities. Even as little as 0.05% of carbonaceous matter darkens sulfur significantly.

Figure 1.1 : A piece of sulfur burning with a blue flame that can hardly be seen at daylight, but it can be seen in this photograph if you look closely. Image by Johannes Hemmerlein and used with permission.
Most sulfur is recovered directly as the element from underground deposits by injecting super-heated water and piping out molten sulfur (sulfur melts at 112o C). Compared to other elements, sulfur has the most allotropes. While the S8 ring is the most common allotrope, there are 6 other structures with up to 20 sulfur atoms per ring.
- Under appropriate conditions, sulfur vapor can contain S
, S2, S4, S6, and S8
- .
- At room temperature, rhombic sulfur (Sα) is a stable solid comprised of cyclic S8
- molecules.
- At 95.5 °C, rhombic sulfur becomes monoclinic sulfur (Sβ). The crystal structure found in monoclinic sulfur differs from that of rhombic sulfur. Monoclinic sulfur is also made up of S8
- molecules.
- Monoclinic sulfur becomes liquid sulfur (Sλ) at 119 °C. Liquid sulfur is straw-colored liquid made up of S8
- molecules and other cyclic molecules containing a range of six to twenty atoms.
- At 160 oC, this becomes a dark, viscous liquid called Liquid sulfur (Sμ). The molecules are still made up of eight Sulfur atoms but the molecule opens up and transforms from a circle into a long spiral-chain molecule.
- At 180 °C, the chain length and viscosity reach their maximum. Chains break and viscosity decreases at temperatures that exceed 180 °C.
- Sulfur vapor is produced when liquid boils at 445 °C. In the vapor that is produced, S8
- molecules dominate but as the vapor continues to heat up, the molecules break up into smaller groups of Sulfur.
- To produce plastic sulfur, Sis poured into cold water. Plastic sulfur is rubberlike and is made up of long, spiral-chain molecules. If plastic sulfur sits for long, it will reconvert to rhombic sulfur.
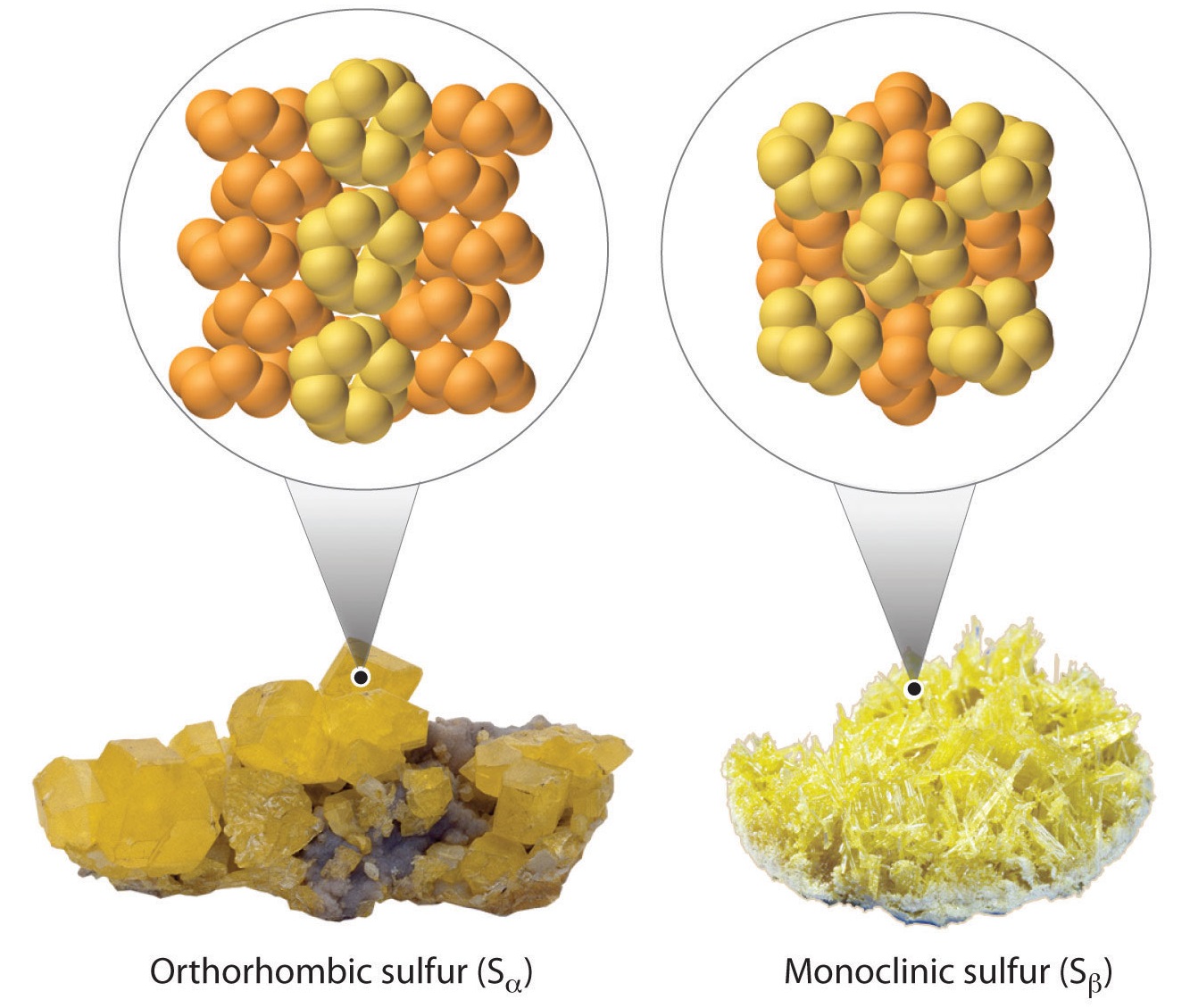
Figure 1.2 : Two allotropes of sulfur.
While oxygen has fewer allotropes than sulfur, including O , O2, O3, O4, O8, metallic O
(and four other solid phases), many of these actually have a corresponding sulfur variant. However, sulfur has more tendency to catenate (the linkage of atoms of the same element into longer chains). Here are the values of the single and double bond enthalpies:
This means that O=O is stronger than S=S, while O–O is weaker than S–S. So, in sulfur, single bonds are favored and catenation is easier than in oxygen compounds. It seems that the reason for the weaker S=S double bonds has its roots in the size of the atom: it's harder for the two atoms to come at a small enough distance, so that the p orbitals overlap is small and the π bond is weak. This is attested by looking down the periodic table: Se=Se has an even weaker bond enthalpy of 272kJ/mol .
What happens when the solid sulfur melts? The S8
molecules break up. When suddenly cooled, long chain molecules are formed in the plastic sulfur which behave as rubber. Plastic sulfur transform into rhombic sulfur over time.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


