
Reduction of Nitro Groups and Aryl Ketones
 المؤلف:
Charge DistributionWilliam Reusch
المؤلف:
Charge DistributionWilliam Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 28-7-2018
28-7-2018
 1922
1922
Reduction of Nitro Groups and Aryl Ketones
Electrophilic nitration and Friedel-Crafts acylation reactions introduce deactivating, meta-directing substituents on an aromatic ring. The attached atoms are in a high oxidation state, and their reduction converts these electron withdrawing functions into electron donating amino and alkyl groups. Reduction is easily achieved either by catalytic hydrogenation (H2 + catalyst), or with reducing metals in acid. Examples of these reductions are shown here, equation 6 demonstrating the simultaneous reduction of both functions. Note that the butylbenzene product in equation 4 cannot be generated by direct Friedel-Crafts alkylation due to carbocation rearrangement. The zinc used in ketone reductions, such as 5, is usually activated by alloying with mercury (a process known as amalgamation).
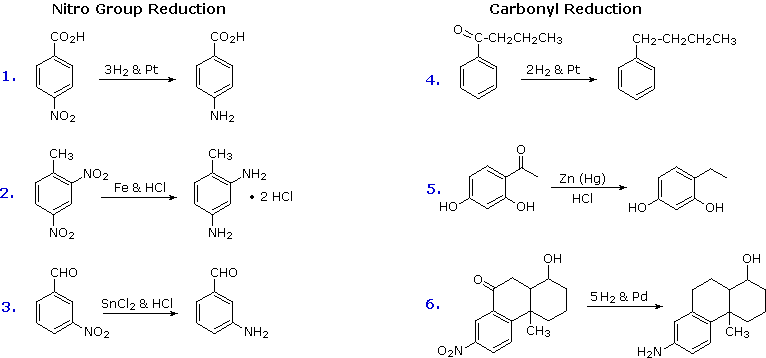
Several alternative methods for reducing nitro groups to amines are known. These include zinc or tin in dilute mineral acid, and sodium sulfide in ammonium hydroxide solution. The procedures described above are sufficient for most cases.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


