


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-12-2018
Date: 5-12-2018
Date: 26-12-2018
|
Reactivity of Dihydrogen
Dihydrogen is not very reactive under ambient conditions, but the lack of reactivity is kinetic (rather than thermodynamic) in origin, and arises from the strength of the H-H bond (Table 1.1). The branching-chain reaction of H2 and O2 is initiated by sparking and the resulting explosion (or ‘pop’ on a small scale) is well known in the qualitative test for H2. Part of the reaction scheme is given (in a simplified form) efficient branching results in a rapid, explosive reaction, and is the reason why it is effective in rocket fuels.
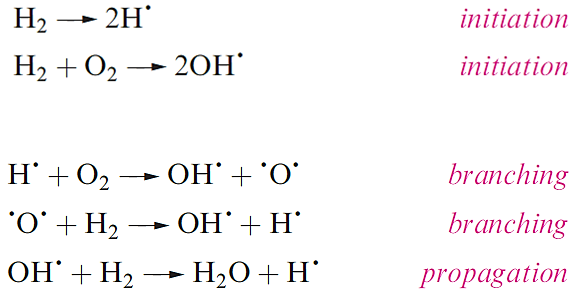
Halogens react with H2 with the ease ofreaction decreasing down group 17. Even at low temperatures, F2 reacts explosively with H2 in a radical chain reaction. In the light-induced reaction of Cl2 and H2, the initiation step is the homolytic cleavage of the Cl_Cl bond to give Cl- radicals which react with H2 to give H- and HCl in one of a series of steps in the radical chain; HCl can be formed in either a propagation or a termination step.

Reactions of H2 with Br2 or I2 occur only at higher temperatures and also involve the initial fission of the X2 molecule. For Br2 (but not for I2) the mechanism is a radical chain .
Table 1.1 Selected physical properties of H2

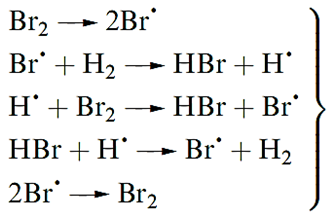



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|