


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-9-2017
Date: 19-1-2016
Date: 13-8-2017
|
Aromatization.
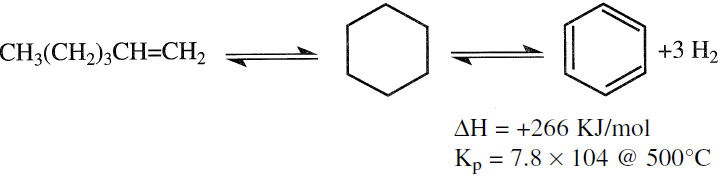
The two reactions directly responsible for enriching naphtha with aromatics are the dehydrogenation of naphthenes and the dehydrocyclization of paraffins. The first reaction can be represented by the dehydrogenation of cyclohexane to benzene.

This reaction is fast; it reaches equilibrium quickly. The reaction is also reversible, highly endothermic, and the equilibrium constant is quite large (6 × l05 @ 500°C).
It is evident that the yield of aromatics (benzene) is favored at higher temperatures and lower pressures. The effect of decreasing H2 partial pressure is even more pronounced in shifting the equilibrium to the right.
The second aromatization reaction is the dehydrocyclization of paraffins to aromatics. For example, if n-hexane represents this reaction, the first step would be to dehydrogenate the hexane molecule over the platinum surface, giving 1-hexene (2- or 3-hexenes are also possible isomers, but cyclization to a cyclohexane ring may occur through a different mechanism). Cyclohexane then dehydrogenates to benzene.
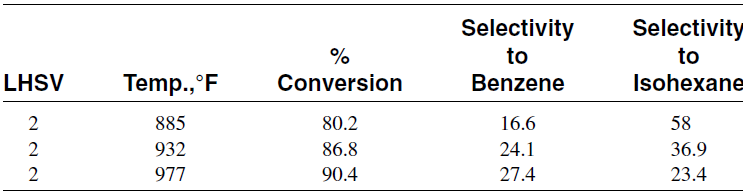
This is also an endothermic reaction, and the equilibrium production of aromatics is favored at higher temperatures and lower pressures. However, the relative rate of this reaction is much lower than the dehydrogenationof cyclohexanes. Table 1.1 shows the effect of temperature on the selectivity to benzene when reforming n-hexane using a platinum catalyst.
Table 1.1 Selectivity to benzene from reforming n-hexane over a platinum catalyst
More often than what has been mentioned above regarding the cyclization of paraffins over the platinum catalyst, the formed olefin species reacts with the acid catalyst forming a carbocation. Carbocation formation may occur by abstraction of a hydride ion from any position along the hydrocarbon chain. However, if the carbocation intermediate has the right configuration, cyclization occurs. For example, cyclization of 1-heptene over the alumina catalyst can occur by the following successive steps:

The formed methylcyclohexane carbocation eliminates a proton, yielding 3-methylcyclohexene. 3-Methylcyclohexene can either dehydrogenate over the platinum surface or form a new carbocation by losing H– over the acid catalyst surface. This step is fast, because an allylic carbonium ion is formed. Losing a proton on a Lewis base site produces methyl cyclohexadiene. This sequence of carbocation formation, followed by loss of a proton, continues till the final formation of toluene.

It should be noted that both reactions leading to aromatics (dehydrogenation of naphthenes and dehydrocyclization of paraffins) produce hydrogen and are favored at lower hydrogen partial pressure.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|