


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-4-2019
Date: 31-12-2015
Date: 1-1-2017
|
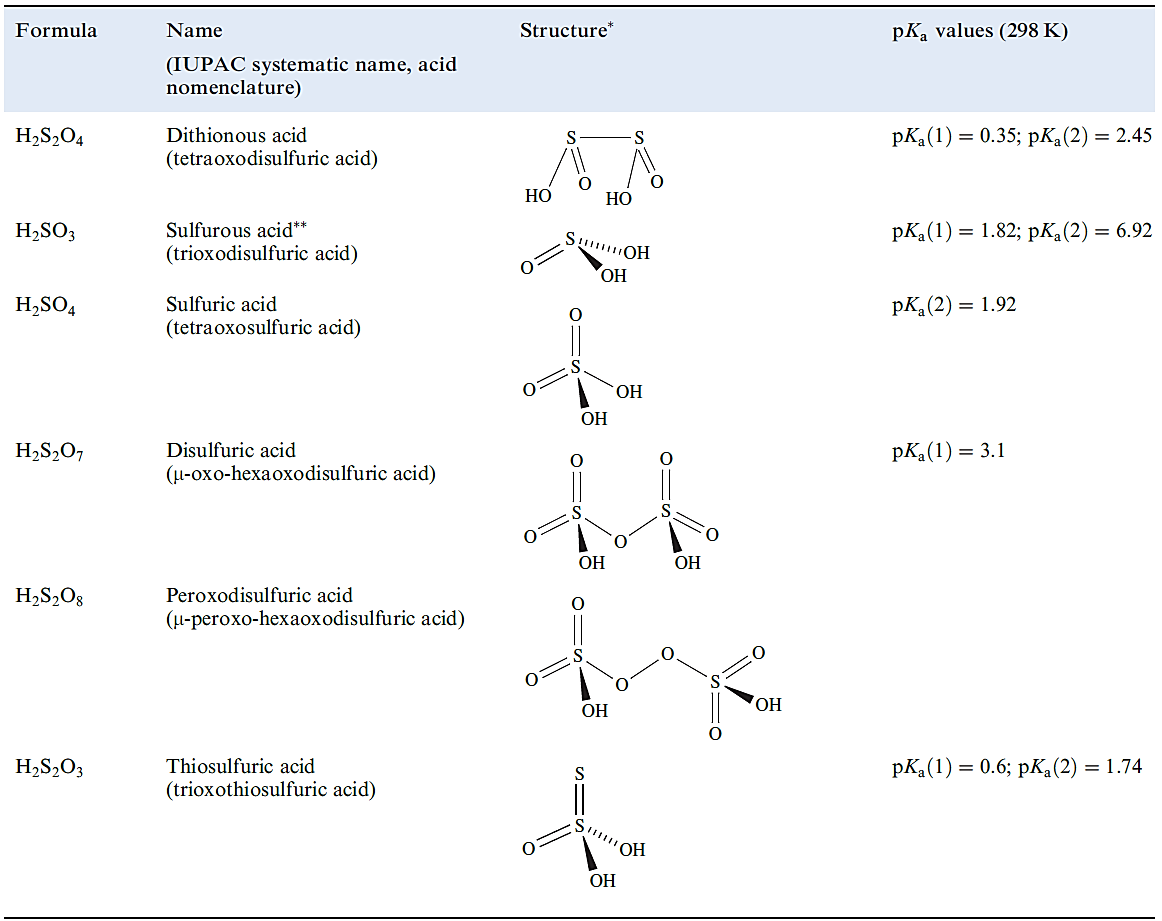
Sulfurous and disulfurous acids, H2SO3 and H2S2O5
Neither ‘sulfurous acid’ nor ‘disulfurous acid’ has been isolated as a free acid. Salts containing the sulfite ion, [SO3]2- , are well established (e.g. β-Na2SO3 and K2SO3 are commercially available) and are quite good reducing agents . Applications of sulfites include those as food preservatives, e.g. an additive in wines . The [SO3]2- ion has a trigonal pyramidal structure with delocalized bonding (S_O = 151 pm, ∠O_S_O = 106 0). There is evidence from 17O NMR spectroscopic data that protonation of [SO3]2- occurs to give a mixture of isomers as shown in equilibrium 1.1.
 (1.1)
(1.1)
Although the [HSO3]- ion exists in solution, and salts such as NaHSO3 (used as a bleaching agent) may be isolated, evaporation of a solution of NaHSO3 which has been saturated with SO2 results in the formation of Na2S2O5 (equation 1.2).
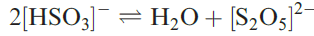 (1.2)
(1.2)
Table 1.1 Selected oxoacids of sulfur.
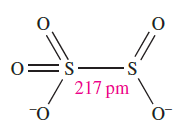
(1.1)
The [S2O5]2- ion is the only known derived anion of disulfurous acid and possesses structure 1.1 with a long, weak S_S bond.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|