


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Introduction to Total and thermotolerant coliforms and Escherichia coli |
|
|
|
Read More
Date: 18-3-2016
Date: 3-3-2016
Date: 9-3-2016
|
Introduction to Total and thermotolerant coliforms and Escherichia coli
1.1 Definition of total coliforms
The group of total coliforms is a subgroup of the Enterobacteriaceae family, which in the 2nd Edition of Bergey’s Manual of Systematic Bacteriology (Brenner and Farmer III, 2005) includes 44 genera and 176 species. The total coliforms group comprises only Enterobacteriaceae capable of fermenting lactose with the production of gas, in 24 to 48 hours at 35°C. More than 20 species fit this definition, among which one can find not only bacteria that originate from the gastrointestinal tract of humans and other hot-blooded animals ( Escherichia coli), but also non-enteric bacteria ( Citrobacter, Enterobacter, Klebsiella and Serratia species, among others).
The ability to ferment lactose can be verified by the formation of gas and/or acid, in culture media containing lactose. These characteristics are used in the traditional methods to enumerate total coliforms. The most modern methods directly detect the activity of the β-galactosidase enzyme, which is involved in the fermentative metabolism of lactose, incorporating substrates for the enzyme in the culture medium. One of these substrates is ONPG ( ortho-nitrophenyl-β-D-galactopyranoside) which, when degraded by β-galactosidase, results in a yellow reaction product. Other substrates are X-GAL (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), which results in an intensely blue reaction and Salmon-Gal ( 6-chloro-3-indolyl-β-D-galactopyranoside), which results in a salmon-pink to red color.
1.2 Definition of thermotolerant coliforms
The group of thermotolerant coliforms, commonly called fecal coliforms, is a subgroup of the total coliform group and includes only members that are capable of fermenting lactose in 24 hours at 44.5–45.5°C, with the production of gas. The objective of this definition was, in principle, to select only Enterobacteriaceae originating from the gastrointestinal tract (E. coli), but it is known that the group also includes members of non-fecal origin (several strains of Klebsiella pneumoniae, Pantoea agglomerans, Enterobacter aerogenes, Enterobacter cloacae and Citrobacter freundii). Because of this, the term “fecal coliforms” has been gradually substituted by “thermotolerant coliforms”
1.3 Escherichia coli
E. coli is included both in the group of total coliforms as in that of thermotolerant coliforms. Its natural habitat is the intestinal tract of hot-blooded animals, although it may also be introduced into foods via non-fecal sources. E. coli is traditionally distinguished from the other thermotolerant coliforms by its growth characteristics in L-EMB Agar ( Levine’s Eosine Methylene Blue Agar) and the profile of the results when subjected to the indole, methyl red, Voges Proskauer and citrate ( IMVC) tests. The most modern methods differentiate E. coli by verifying the activity of the β-glucuronidase enzyme, produced by 96% of the E. coli strains, including the anaerogenic (i.e. non-gas producing) strains (Feng and Hartman, 1982). One of the substrates utilized to verify the activity of β-glucuronidase is MUG (4-methylumbelliferyl-β-D-glucuronide), which, when degraded by β-glucuronidase, results in 4-methyl-umbelliferone, which is fluorescent under UV light.
Another substrate commonly used to this purpose is BCIG (5-bromo-4-chloro-3-indolyl-β-D-glucuronide), also called X-β-D-Glucuronide, which when degraded by the enzyme, forms a blue reaction product.
1.4 Use as indicators
According Kornacki and Johnson (2001) E. coli was initially introduced as an indicator in 1892 in Australia and in 1895 in the United States. It was used to indicate the contamination of water by fecal material and, consequently, to alert for the potential presence of enteric pathogens ( Salmonella, for example). The standard was changed to total coliforms in 1915, by the U.S. Public Health Service, based on the (questionable) premise that all coliforms were of equal value as indicators of fecal contamination. After their use as indicators of the microbiological quality of water, they began to be utilized for the same purpose for foods in general, without any judicious and thorough evaluation of the validity of their use to this purpose in different products. At present, the premise that there is a direct correlation between high numbers of E. coli, thermotolerant coliforms, total coliforms and Enterobacteriaceae in foods with fecal contamination is no longer valid, for a series of reasons: 1) E. coli, thermotolerant coliforms, total coliforms or Enterobacteriaceae are not obligate inhabit-ants of the intestinal tract of hot-blooded animals, and can be found in a number of different environmental reservoirs. 2) The presence of these microorganisms is common in food processing environments, and may even become part of the resident microbiota of the facility (especially when cleaning and sanitation conditions are inadequate). 3) Several strains of E. coli, coliforms or Enterobacteriaceae may grow in refrigerated foods.
Based on these facts, the Food and Agricultural Organization and the World Health Organization (FAO/WHO, 1979) concluded that it is not possible to assess the safety (innocuity) of foods as a function of the levels of E. coli, thermotolerant coliforms, total coliforms or Enterobacteriaceae. High levels of these microorganisms may, under certain circumstances, be related to or associated with a greater probability of the presence of enteric pathogens, however, frequently this is not the case. In the same way, its absence does not necessarily mean that the products are free from enteric pathogenic bacteria. Kornacki and Johnson (2001) listed the following applications for these microorganisms as indicators:
a) Enterobacteriaceae and coliforms – indicators of the hygienic conditions of manufacturing processes, since they are easily inactivated by sanitizing agents and capable of colonizing several niches in processing plants, when sanitation and cleaning procedures are inappropriate or inadequately executed.
b) Coliforms – indicators of processing flaws or post-processing contamination of pasteurized foods, since they are easily destroyed by heat and do not survive heat treatment.
c) E. coli – indicator of fecal contamination in fresh (“in natura”) foods (but not in processed foods).
1.5 Methods of analysis
Classical MPN method: The classical method to per-form total coliforms, thermotolerant coliforms and E. coli counts in water and foods is the Most Probable
Number (MPN) technique, which includes the follow-ing steps: 1°) Presumptive test, in which three aliquots of three dilutions of the sample are inoculated into a series of three tubes containing Lauryl Sulphate Tryptose (LST) broth per dilution. LST contains lactose and the observation of growth accompanied by the production of gas from lactose fermentation, after 24–48 h incubation at 35°C, is considered indicative or suspect (presumptive) of the presence of coliforms. 2°) For the confirmation of total and thermotolerant coliforms, a loopful of each suspected tube is transferred to tubes with Brilliant Green Bile (BGB) Broth 2% and E. coli Broth (EC), selective culture media containing lactose.
The observation of growth with the production of gas in the BGB tubes, after 24–48 h incubation at 35°C, is considered confirmative of total coliforms. Growth with gas production in the EC tubes, after 24 h incubation at 45.5°C (or 44.5°C, in the case of water), is considered confirmative of thermotolerant coliforms. 3°) The EC tubes testing positive for the presence of thermotolerant coliforms are suspected of the presence of E. coli. For confirmation, a loopful of each tube is streaked onto Levine’s Eosine Methylene Blue (L-EMB) Agar, a differential selective medium to distinguish E. coli from other thermotolerant coliforms. If any development of typical E. coli colonies is observed on L-EMB, two of these colonies are to be isolated for the biochemical indole, MR, VP and citrate ( IMVC) assays. The cultures with the + + − − (biotype 1) or − + − − (biotype 2) profiles are considered confirmed.
In the classical MPN method, the last step of the test is optional and many laboratories conclude the analysis with the confirmation of thermotolerant coliforms. When the presence of E. coli is verified, the assay is referred to as “complete test”. In water analysis, the section 921 of the 21st Edition of the Standard Methods for the Examination of Water and Wastewater (Hunt and Rice, 2005) recommends different procedures from those used for the confirmation of E. coli. One of these consists in transferring the suspected cultures obtained in LST to tubes containing EC broth with MUG. After incubation at 44.5ºC/24 h, the cultures showing blue fluorescence under UV light are considered confirmed.
Another procedure is the transference from LST to Tryptone (Tryptophane) Broth, incubation at 44.5ºC/24 h and the indole test. The cultures that are positive in the indole test are considered confirmed.
In the MPN method developed by ISO 7251:2005 for thermotolerant coliforms in foods, incubation in EC broth is done at 44 ± 1ºC and the presumptive confirmation of the presence of E. coli also utilizes the indole test, after growth in Tryptone (Tryptophane) Broth at 44 ± 1ºC. The main advantage of the ISO method is that the acceptable temperature variation is ±1ºC, which can be achieved in laboratory incubators. In the Compendium, the maximum acceptable variation is ±0.2°C, which requires a water bath for incubation. The big problem with this requirement is that water baths with this level of temperature stability are rather expensive, as are the thermometers capable of detecting this variation.
Chromogenic substrate method: For the determination of total coliforms and Escherichia coli in water, an extremely simple and practical method is that of the chromogenic and fluorogenic substrate ( COLIL-ERT®) AOAC 991.15 (Horwitz and Latimer, 2010), a culturing technique based on the addition of a defined or specific and at the same time differential culture medium to the sample, in which the exact balance between all the components ensures the specificity of the result. The medium contains two substrates for enzymes: a) ortho-nitrophenyl-β-D-galactopyranoside (ONPG), a substrate for the β-galactosidase enzyme of coliforms, the reaction product of which is yellow.
b) 4-methylumbelliferyl-β-D-glucuronide ( MUG), a substrate for the β-glucuronidase enzyme of E. coli, the reaction product of which is fluorescent under UV light. The test can be conducted in two ways: a) Presence/absence in 100 mL, by adding the culture medium to 100 ml of the sample (the sterile, dried medium is marketed in ampoules containing the exact quantity necessary for 100 ml samples). b) Most probable Number (MPN) in 100 ml, by dividing the 100 ml into 10 aliquots of 10 ml each.
Plate count method: For the enumeration of total coliforms in foods, the Compendium (Kornacki and Johnson, 2001) and the Standard Methods for the Examination of Dairy Products (Davidson et al., 2004) also recommend the direct plate count method in plates containing Violet Red Bile Agar (VRB). This method is based on the same counting principle as that of the Enterobacteriaceae plate count, described in a specific chapter, but uses lactose instead of glucose, in the VRB Agar.
Petrifilm™ (3M Company). This is a modified version of the Colony Formining Unit (CFU) plate count, and consists of two sterile, rehydratable films impregnated with culture medium and cold-water-soluble-gelling agents. Inoculation is done directly onto the bottom film, which, after inoculation, is covered with the top film. The inoculum is evenly distributed over the circular growth area of the bottom film by gentle manual pressing with a plastic spreader and, after solidification of the gel, the plates are incubated for the development of colonies.
The culture medium that forms the basis of the sys-tem is Violet Red Bile (VRB), selective for enterobacteria, supplemented with triphenyltetrazolium chloride (TTC), an indicator that, when reduced, colors colonies red, thereby facilitating their visualization. The medium contains lactose, which, fermented by coliforms or E. coli, produces gas bubbles around the colonies. Typical colonies appear red surrounded by gas bubbles. In the petrifilm version for coliforms + E. coli, the medium also contains BCIG, a chromogenic substrate for the β-glucuronidase enzyme, which allows to differentiate E. coli by the formation of a blue precipitate sur-rounding the colonies. The high sensivity petrifilm version (High-Sensitivity Coliform Count Plate) allows the inoculation of volumes of 5 ml while the petrifilm version for rapid counts (Rapid Coliform Count Plate) allows to obtain the result within 6 to 14 h.
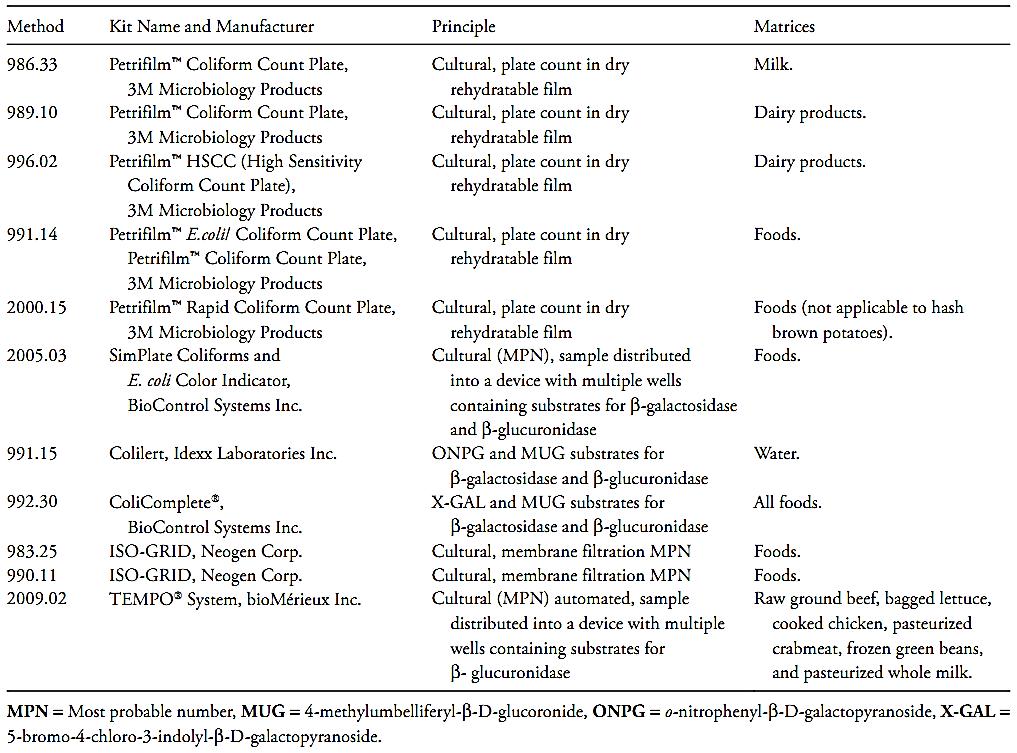
Other methods that already have been officially recognized by the AOAC International are the microbiological test kits described in Table 1.
Table 1 Analytical kits adopted as AOAC Official Methods for coliforms and E. coli in foods (Horwitz and Latimer, 2010, AOAC Inter-national, 2010).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Brenner, D.J. & Farmer III, J.J. (2005) Family I. Enterobacteriaceae. In: Brenner, D.J., Krieg, N.R. & Staley, J.T. (eds). Bergey’s Manual of Systematic Bacteriology. Volume 2. 2nd edition. New York, Springer Science+Business Media Inc. pp. 587–607.
Kornacki, J.L. & Johnson, J.L. (2001) Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbio-logical Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 8, pp. 69–82.
FAO/WHO (1979) Report of a joint FAO/WHO Working Group on Microbiological Criteria for Foods. Food and Agricultural Organi-zation and the World Health Organization. Report number: Document WG/Microbiol./79/1.
Feng, P.C.S. & Hartman, P.A. (1982) Fluorogenic assay for immediate confirmation of Eschericihia coli. Applied and Environmental Microbiology, 43, 1320–1329.
Hunt, M.E. & Rice, E.W. (2005) Microbiological examination. In: Eaton, A.D., Clesceri, L.S., Rice, E.W. & Greenberg, A.E. (eds). Standard Methods for the Examination of Water & Wastewater. 21st edition. Washington, American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF). Part 9000, pp. 9.1–9.169.
Davidson, P.M., Roth, L.A. & Gambrel-Lenarz, S.A. (2004) Coliform and other indicator bacteria. In: Wehr, H.M. & Frank, J.F (eds). Standard Methods for the Examination of Dairy Products. 17th edition. Washington, American Public Health Association. Chapter 7, pp. 187–226.
Horwitz, W. & Latimer, G.W. (eds) (2010) Official Methods of Analysis of AOAC International. 18th edition., revision 3. Gaithersburg, Maryland, AOAC International.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|