


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-12-2020
Date: 24-12-2020
Date: 29-2-2016
|
Diphtheria
Caused by Corynebacterium diphtheriae toxin vaccines against diphtheria immunization of children with diphtheria toxoid is now universal in most of the countries of the world .except in rare circumstances ,Diphtheria toxoid is no longer administrated as a single antigen but is combined with tetanus toxoid and Bordetella pertussis vaccine .The combined form is designated as DPT or triple vaccine .The tetanus and diphtheria toxoid are often adsorbed on insoluble aluminum salts .The B .pertussis component act as adjuvant and enhances the antitoxin response to both toxoids .Different schedules have been recommended in different countries but all aim at attaining level of 0.001 unit / of antitoxin which is considered adequate protection against infection with a toxigenic strain of C. diphtheriae
Immunization schedules for DPT vaccines
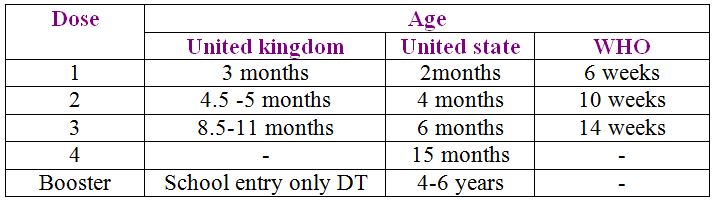
Diphtheria toxoid this prepared from diphtheria toxin liberated by standard strain of Corynebacterium diphtheriae (PW8) by detoxifiying the toxin with formaldehyde .Single human dose of diphtheria toxoid contains no more than 30 LF of toxoid preservative (other than phenol ) can be added if vaccine is to be dispensed in multidose containers ,and an adjuvant (aluminum or calcium compounds ) is generally used as amineral carrier with concentration of aluminium not exceeding 1.25 mg and that of calicium 1.3 mg per single human dose .
Storage :
The diphtheria toxoid should not be frozen and storage at 2-8 C has been found to be satisfactory .Once frozen ,this toxoid should not be used .The presence of granular or flaky particlesin the vaccine or the formation of deposit below a column of transparent fluid within 30 minutes of shaking is an indication that the vaccine has been frozen
Dose and dose schedules
Single dose is of 0.5 ml injected subcutaneously or intramuscularly the diphtheria toxoid is given in combination with tetanus DT or tetanus and pertussis antigens DPT( triple vaccine ) three doses are adequate when given at intervals of 4 weeks starting from 6 weeks of life .Booster dose is needed at 18 months of age and may be given against at school entry age.
Adverse reactions
Allergic phenomenon every type of allergic response can be elicited in human following injection with diphtheria toxoid .Anaphylaxis arthus reactions as well as delayed type of hypersensitivity reaction have been shown with this vaccine .
contra indication
1- Acute febrile illness 2- sever reactions such as neurological or anaphylactic reactions to an earlier diphtheria immunization
Assessing efficacy of vaccine
It can be done either by the measured of antidiphtheria toxin in the serum of the vaccine or by performing schick test a level of › 0.02 IU/ml of antitoxin in the serum of vaccine shell indicate adequate protection .Schick test is an in vivo skin test where in purified diphtheria toxin 0.2 ml is injected intradermally in a child of more than 2 months of age and readings are taken after 24-48 hours and 5-7 days .Absence of a reaction indicates immunity to diphtheria
Future prospects
pertussis / the name whooping cough caused by Bordetella pertussis vaccines against pertussis
whole cell vaccine is prepared from one or more strains of Bordetella pertussis .Strians are chosen in such away that the final vaccine includes agglutinogens 1,2 and 3. In the final bulk the concentration of bacteria for each human dose of 0.5 ml corresponds to an opacity before killing of not more than 40 IU .Each bulk is examined for the presence of agglutinogens 1,2 and 3before the addition of adjuvant .the concentration of aluminum as an adjuvant ,does not exceed 1.25 mg and that of calcium 1.3 mg per asingle human dose .A preservative other than phenol is added
Storage :
the vaccine is not frozen and storage at 2-8 C is adequate .The expiry date is not more than 2.5 years from the date last potency test was carried out with satisfactory results
Dose and immunizing schedule
Pertussis vaccine is not available as single antigen .Instead it is always injected as apart of DPT (triple) ,the required potency is present in 0.5 ml of DPT .The schedule is also same as for other components given collectively .The vaccine is injected subcutaneously or intramuscularly .An interval of minimum four weeks is mandatory between two doses of DPT .
Immunity :Does not produce a life long immunity about 2 years of complete course
Adverse reactions
Majority of the adverse reactions seen with DPT occur because of the pertussis component
In the UK a national childhood encephalopathy study was established in 1976 to determine the detrimental effects of vaccine . This was a case control study and concluded that.
A cellular pertussis vaccines Japans towards the development of an improved pertussis vaccine with only those components which induce immunity and do not cause adverse effects .The first vaccine that was designed specifically to contain known components of Bordetella pertussis in identifiable Quantities was that described by Sato and coworkers .this vaccine was also known as first generations a cellular pertussis vaccine and has given rise to possible three more generations of a cellular pertussis vaccines

PT=pertussis toxoid
rDNA = recombinant DNA technology



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|