


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 10-3-2021
Date: 5-4-2021
Date: 17-6-2021
|
The S. cerevisiae Centromere Binds a Protein Complex
KEY CONCEPTS
-A specialized protein complex that is an alternative to the usual chromatin structure is formed at CDE-II.
-The histone H3 variant Cse4 is incorporated in the centromeric nucleosome.
-The CBF3 protein complex that binds to CDE-III is essential for centromeric function.
-The proteins that bind CEN serve as an assembly platform for the kinetochore and provide the connection to microtubules.
Can we identify proteins that are necessary for the function of CEN sequences? There are several genes in which mutations affect chromosome segregation and whose proteins are localized at centromeres. FIGURE 1 summarizes the contributions of these proteins to the centromeric structure.
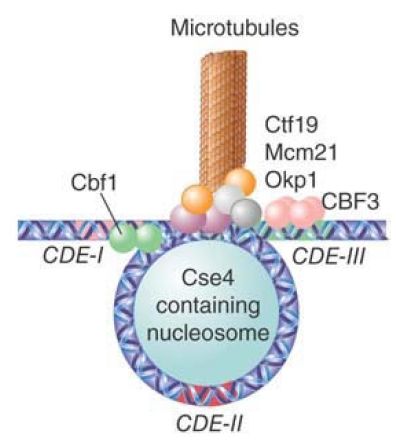
FIGURE 1 The DNA at CDE-II is wound around an alternative nucleosome containing Cse4, CDE-III is bound by the CBF3 complex, and CDE-I is bound by a Cbf1 homodimer. These proteins are connected by the group of Ctf19, Mcm21, and Okp1 proteins, and numerous other factors serve to link this complex to a microtubule.
The CEN region recruits three DNA-binding factors: Cbf1, CBF3 (an essential four-protein complex), and Mif2 (CENP-C in multicellular eukaryotes). In addition, a specialized chromatin
structure is built by binding the CDE-II region to a protein called Cse4, a histone H3 variant (analogous to CENP-A in multicellular eukaryotes), probably in the context of an otherwise normal nucleosome. A protein called Scm3 is required for proper association of Cse4 with CEN. Inclusion of CenH3 histone variants related to Cse4 is a universal aspect of centromere construction in all species. The basic interaction consists of bending the DNA of the CDE-II region around a protein aggregate; the reaction is probably assisted by the occurrence of intrinsic bending in the CDE-II sequence.
CDE-I is bound by a homodimer of Cbf1; this interaction is not essential for centromere function, but in its absence the fidelity of chromosome segregation is reduced about 10×. The 240-kD heterotetramer, CBF3, binds to CDE-III. This interaction is essential for centromeric function.
The proteins bound at CDE-I, CDE-II, and CDE-III also interact with another group of proteins (Ctf19, Mcm21, and Okp1), which in turn link the centromeric complex to the kinetochore proteins (at least 70 individual kinetochore proteins have been identified in yeast) and to the microtubule.
The overall model suggests that the complex is localized at the centromere by a protein structure that resembles the normal building block of chromatin (the nucleosome). The bending of DNA at this structure allows proteins bound to the flanking elements to become part of a single complex. The DNA-binding components of the complex form a scaffold for assembly of the kinetochore, linking the centromere to the microtubule. The construction of kinetochores follows a similar pattern, and uses related components, in a wide variety of organisms.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|