


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-7-2018
Date: 12-7-2018
Date: 14-9-2020
|
The double bond of an alkene consists of a sigma (σ) bond and a pi (π) bond. Because the carbon-carbon π bond is relatively weak, it is quite reactive and can be easily broken and reagents can be added to carbon. Reagents are added through the formation of single bonds to carbon in an addition reaction.

One important alkene addition reaction is hydrogenation., where the alkene undergoes reduction to an alkane. In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In other words, the energy of the product is lower than the energy of the reactant; thus it is exothermic (heat is released). The heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability.
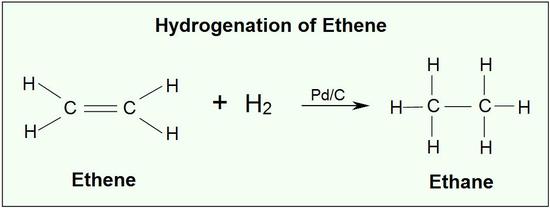
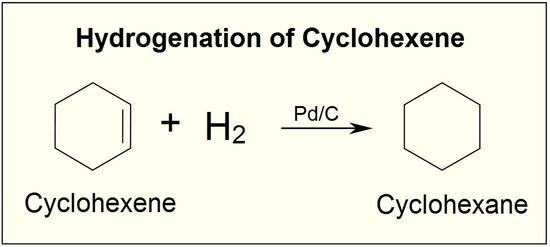
Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst.

Common catalysts used are insoluble metals such as palladium in the form Pd-C, platinum in the form PtO2, and nickel in the form Ra-Ni. With the presence of a metal catalyst, the H-H bond in H2 cleaves, and each hydrogen attaches to the metal catalyst surface, forming metal-hydrogen bonds. The metal catalyst also absorbs the alkene onto its surface. A hydrogen atom is then transferred to the alkene, forming a new C-H bond. A second hydrogen atom is transferred forming another C-H bond. At this point, two hydrogens have added to the carbons across the double bond.

Because this reaction takes place on a planar surface, addition of hydrogen occurs on the same face of the double bond – a syn addition, in other words. The catalytic hydrogenation of 1,2-dimethylcyclopentane will yield, for example, the cis dimethylcycloalkane product, with little or no formation of a trans product.

Other double bonds such as C=O can also be hydrogenated, but this reduction is usually slower than for hydrogenation of alkenes. This fact can be used to advantage in carrying out selective reactions. For instance, hydrogenation of a carbon-carbon double bond can be achieved without simultaneously reducing a carbonyl bond in the same molecule. For example the carbon-carbon double bond of the following aldehyde can be reduced selectively:

Hydrogenation reactions are extensively used to create commercial goods. Hydrogenation is used in the food industry to make a large variety of manufactured goods, like spreads and shortenings, from liquid oils. This process also increases the chemical stability of products and yields semi-solid products like margarine. Hydrogenation is also used in coal processing. Solid coal is converted to a liquid through the addition of hydrogen. Liquefying coal makes it available to be used as fuel.



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
علي بابا تطلق نماذج "Qwen" الجديدة في أحدث اختراق صيني لمجال الذكاء الاصطناعي مفتوح المصدر
|
|
|
|
|
|
|
ضمن برنامج تأهيل المنتسبين الجدد قسم الشؤون الدينية يقدم محاضرات فقهية وعقائدية لنحو 130 منتسبًا
|
|
|