
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-5-2019
Date: 18-11-2019
Date: 1-9-2019
|
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is eliminated in the reaction, which is acid-catalyzed and reversible in the same sense as acetal formation. The pH for reactions which form imine compounds must be carefully controlled. The rate at which these imine compounds are formed is generally greatest near a pH of 5, and drops at higher and lower pH's. At high pH there will not be enough acid to protonate the OH in the intermediate to allow for removal as H2O. At low pH most of the amine reactant will be tied up as its ammonium conjugate acid and will become non-nucleophilic.
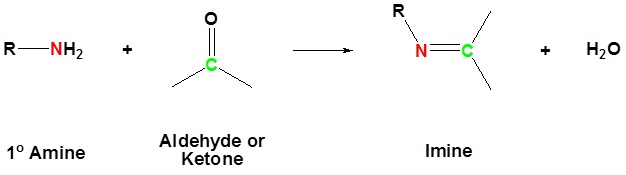
Converting reactants to products simply
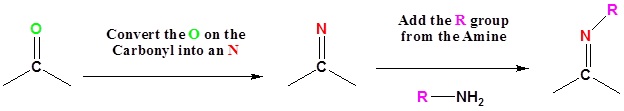



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
هيأة المساءلة والعدالة: مؤتمر ذاكرة الألم يوثّق سنوات القهر التي عاشها العراقيون إبّان الحكم الجائر
|
|
|