We have daily contact with many transition metals. Iron occurs everywhere—from the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Chromium is useful as a protective plating on plumbing fixtures and automotive detailing.
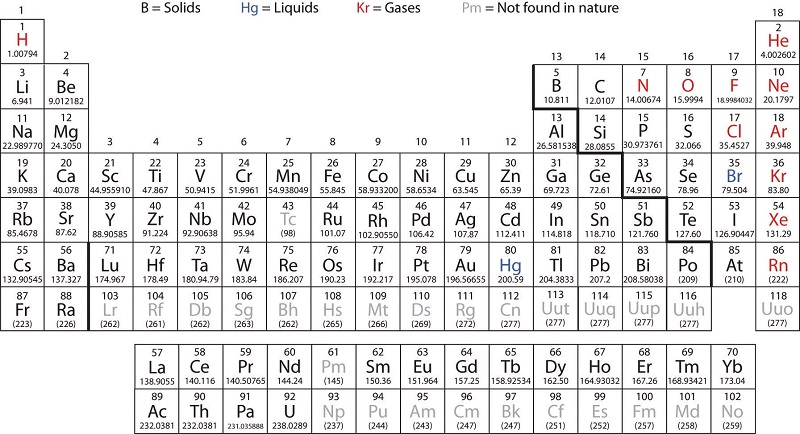
Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. As shown in Figure 1.2, the d-block elements in groups 3–11 are transition elements. The f-block elements, also called inner transition metals (the lanthanides and actinides), also meet this criterion because the d orbital is partially occupied before the f orbitals. The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. However, the group 12 elements do display some of the same chemical properties and are commonly included in discussions of transition metals. Some chemists do treat the group 12 elements as transition metals.