
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-1-2017
Date: 2-2-2018
Date: 5-7-2020
|
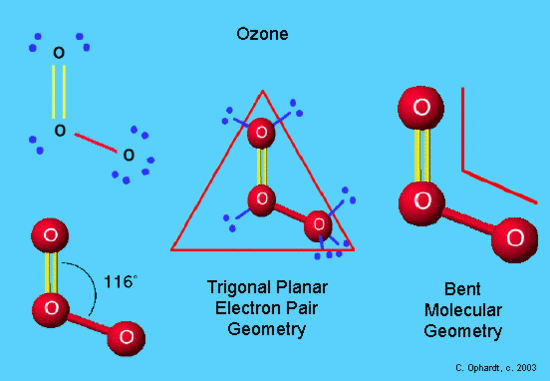
In this example, O3, the Lewis diagram shows O at the center with one lone electron pair and two other oxygen atoms attached. This shows trigonal planar for the electron pair geometry and and bent the molecular geometry. The one lone electron pair exerts a little extra repulsion on the two bonding oxygen atoms to create a slight compression to a 116obond angle from the ideal of 120o. Compare it to the beryllium hydride (BeH2) which has 2 hydrogen atoms and no lone electron pairs. Ozone is a protective molecule by absorbing UV radiation when high in the stratosphere. However when close to the surface, ozone is produced as part of photochemical smog air pollution and is a powerful lung irritant.



|
|
|
|
مقاومة الأنسولين.. أعراض خفية ومضاعفات خطيرة
|
|
|
|
|
|
|
أمل جديد في علاج ألزهايمر.. اكتشاف إنزيم جديد يساهم في التدهور المعرفي ؟
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقيم ندوة علمية عن روايات كتاب نهج البلاغة
|
|
|